Tracheostomy and Ventilator Dependence
The scope of this page includes communication and swallowing disorders in patients with tracheostomy tubes or endotracheal tubes (ETTs), both with and without mechanical ventilator dependence, across the life span.
See the Tracheostomy and Ventilator Dependence Evidence Map for summaries of the available research on this topic.
Speech-language pathologists (SLPs) with appropriate training contribute to the communication and swallow assessment and management of patients with tracheostomy tubes or ETTs, both with and without mechanical ventilator dependence, in cooperation with an interprofessional team.
A tracheotomy is a surgical procedure that involves an incision in the trachea and placement of a tube to create an artificial airway. A tracheostomy is the opening (tracheostoma) into the trachea created by the tracheotomy through which the tracheostomy tube can be inserted. Tracheostomy tubes can also be inserted using a technique called percutaneous dilation tracheostomy. A tracheostomy tube is a curved tube inserted into the tracheostoma to maintain an airway. An ETT is a tube that is inserted through the mouth that passes through the vocal folds into the trachea to maintain an airway. Some tracheostomy patients, and most ETT patients, require mechanical ventilation, a form of ventilation that uses a machine to help deliver oxygen to a patient. This machine may also help remove carbon dioxide.
Patients have diverse experiences in the type and severity of communication and swallowing difficulties due to the wide variety of medical conditions that may necessitate a tracheostomy (with or without mechanical ventilation). Individualized assessment and management require interprofessional collaborative practice. A tracheostomy team may include (but is not limited to) an otolaryngologist, a pulmonologist, a respiratory therapist, nurses, and an SLP.
This team may consult with additional professionals (e.g., physical therapists and occupational therapists) as necessary. SLPs may have to advocate for their inclusion on teams in facilities where their expertise with trach/vent patients is underutilized (S.D. Davis et al., 2021; Freeman-Sanderson et al., 2011).
Positive patient and health care organizational outcomes—including reductions in cannulation times, shorter duration to communication, hospital length of stay, adverse events, and cost of care—have been realized when patients with tracheostomy are managed with a multidisciplinary team approach (Bonvento et al., 2017; Brenner et al., 2020; Chorney et al., 2021; Ninan et al., 2023; Whitmore et al., 2020). See ASHA’s resource on interprofessional education/interprofessional practice (IPE/IPP).
Other ASHA Practice Portal pages that are applicable to this topic include Adult Dysphagia, Pediatric Feeding and Swallowing, Voice Disorders, Head and Neck Cancer, and Augmentative and Alternative Communication.
Incidence and Prevalence
Incidence of tracheostomy refers to the number of new cases identified with new tracheostomy in a specified time period.
Prevalence of tracheostomy refers to the number of people living with tracheostomy in a given time period.
In the United States, approximately 40% of pediatric and 20%–40% of adult intensive care unit (ICU) patients require mechanical ventilation (Society of Critical Care Medicine, n.d.). Of those who require ventilation, 6.2% of individuals require it for a prolonged period of time (i.e., over 21 days of ventilation for at least 6 hours a day; Lone & Walsh, 2011).
The annual incidence rate for tracheostomy placements is approximately 28.4–39.7 cases out of every 100,000 adults in the United States (Abril et al., 2021). Pediatric tracheostomies occur much less frequently, with a reported annual incidence rate of 6.0–7.1 cases per 100,000 individuals under the age of 18 years (Muller et al., 2019). Individual statistics regarding the rates of tracheostomy within specific diagnoses are not readily available.
Estimates of the incidence and prevalence of tracheostomy seen in commonly associated clinical populations are as follows:
Acquired brain injury: Approximately 21%–47% of mechanically ventilated adults with severe acquired brain injury require tracheostomy (Wahlster et al., 2021).
Acute respiratory distress syndrome (ARDS): It is estimated that 5%–15% of adults with ARDS admitted to the ICU require tracheostomy (Abe et al., 2018; Wahlster et al., 2021).
Amyotrophic lateral sclerosis (ALS): It is estimated that 16.4%–31.3% of adults with ALS undergo tracheostomy; however, estimates may differ based upon the degree of disease progression and patient preferences regarding end-of-life decisions (Segura et al., 2023; Spataro et al., 2012).
Burn injuries: Approximately 0.9% of adult patients admitted with burn injuries require tracheostomy (Mourelo et al., 2015), with an increased rate of 30.9% for those requiring ICU-level care (Janik et al., 2021).
COVID-19: Approximately 12.2%–16.4% of adults receiving ICU-level care secondary to COVID-19 require tracheostomy (Mahmood et al., 2021; Martin-Villares et al., 2021).
Head and neck cancer: Reported rates of tracheostomy for adults requiring surgery due to head and neck cancer range from 17% to 20.3% (COVIDSurg Collaborative, 2021; Siddiqui et al., 2016).
Respiratory failure: Each year, an average of 9.1%–9.6% of hospitalized adults requiring mechanical intervention due to respiratory failure receive tracheostomy (Abril et al., 2021; Mehta, Syeda, Bajpayee, et al., 2015).
Traumatic brain injury: Approximately 29%–31.8% of adults with traumatic brain injury receiving ICU-level care require tracheostomy (Pelosi et al., 2011; Robba et al., 2020).
Spinal cord injury (SCI): It is estimated that 13.8%–17.7% of adults with acute traumatic cervical SCI require tracheostomy (Long et al., 2022; Mu & Zhang, 2019).
Stroke: Approximately 1.3%–1.9% of adults require tracheostomy following acute stroke (Chatterjee et al., 2018; Walcott et al., 2014), whereas 14%–20% of adults who require mechanical ventilation status after stroke receive tracheostomy (Pelosi et al., 2011).
Roles and Responsibilities
SLPs play a central role in the screening, assessment, diagnosis, and treatment of persons with swallowing and/or communication disorders related to artificial airways. An artificial airway is a device that is used to facilitate ventilation and secretion management. These include the endotracheal tube (ETT)—a tube placed into the trachea via the mouth or nose to establish and/or maintain the airway and ventilation—and the tracheostomy tube. Artificial airways may be used to access mechanical ventilation. The professional roles and activities in speech-language pathology include clinical/educational services (diagnosis, assessment, planning, and treatment), advocacy, administration, and research. See ASHA’s Scope of Practice in Speech-Language Pathology (ASHA, 2016).
Appropriate roles for SLPs include, but are not limited to, the following.
Assessment
- Identifying the signs and symptoms of dysphagia, communication disorders and delays, and voice disorders.
- Making recommendations for the consideration and appropriateness of cuff deflation trials, capping trials, a one-way speaking valve, or a talking tracheostomy tube.
- Conducting a culturally and linguistically appropriate assessment for swallowing and communication.
- Assessing communication options, including augmentative and alternative communication (AAC).
Counseling and Education
- Educating other professionals on the role of the SLP in the care of patients with tracheostomy, both with and without mechanical ventilator dependence.
- Providing education and counseling about the impact (current or anticipated) of tracheostomy, mechanical ventilation, and one-way speaking valve placement and/or use on communication and swallowing to patients and caregivers, as appropriate.
- Providing training and education regarding the use of AAC.
Treatment
- Collaborating with other professionals (i.e., tracheostomy team) as well as the patient and their care partners regarding the management of swallowing and communication disorders in patients with tracheostomy, both with and without mechanical ventilator dependence.
- Providing safe, effective, and evidence-based treatment for swallowing and communication disorders.
- Providing clinical recommendations on the tracheostomy weaning pathway, including tracheostomy tube selection, tube changes/downsizing, capping trials, and the decannulation process.
- Facilitating safe and effective use of a one-way speaking valve for patients with or without mechanical ventilator dependence or a specialty tracheostomy tube (for patients who are difficult to fit with standard tubes).
Other
- Maintaining general knowledge of the anatomy, physiology, and pathophysiology of the swallowing mechanism and the respiratory system.
- Referring to other professionals as appropriate to ensure comprehensive care and best outcomes.
- Providing counseling toward facilitating a return to school/work in collaboration with the medical team.
- Identifying and using appropriate functional outcome measures.
- Advocating for individuals requiring tracheostomy, both with and without mechanical ventilator dependence.
- Contributing to performance improvement, safety outcomes, and research in communication and swallowing disorders.
Essential Knowledge
Safe assessment and intervention for the patient with a tracheostomy tube, with or without mechanical ventilator dependence, requires knowledge of the following:
- the anatomy, physiology, and pathophysiology of the swallowing mechanism and the respiratory system
- common medical conditions and etiologies associated with tracheostomy and their potential impacts on communication, cognition, swallowing, and overall plans of care
- tracheostomy tube design—including various types, sizes, and components—and rationale for the use of each tube
- reasons for tracheostomy placement
- methods of tracheostomy insertion (i.e., percutaneous vs. surgical)
- potential early and late complications of tracheostomy placement
- knowledge of the different types of tracheostomy equipment
- understanding the functions, settings, and modes of mechanical ventilation as well as optimal ventilator settings and modes for the evaluation and treatment of communication and swallowing deficits
- the physiologic changes in voice, swallowing, and respiration that occur secondary to tracheostomy, both with and without mechanical ventilation
- how to monitor physiological changes during assessment or therapy and how to intervene as needed
- potential psychological and emotional impact of tracheostomy placement
- knowledge of infection control and the proper use of personal protective equipment related to artificial airways, including tracheostomies, with and without mechanical ventilator dependence
- social determinants of health—the nonmedical factors and forces of someone’s daily life that impact their health outcomes
- roles of other professionals (e.g., otolaryngologists) and when to make referrals
As indicated in the ASHA Code of Ethics (ASHA, 2023), SLPs who serve this population should be specifically educated and appropriately trained to do so. It is important to consider potential liability associated with tracheostomy-related procedures, such as deep suctioning, cuff inflation and deflation, and changing or capping tracheotomy tubes.
Populations Requiring Tracheostomy With or Without Mechanical Ventilation
SLPs may encounter patients with tracheostomy, both with and without mechanical ventilator dependence, in a variety of settings—including hospitals, skilled nursing facilities, rehabilitation centers, outpatient clinics, long-term acute care centers, schools, and home health care.
Patient populations requiring a tracheostomy (with or without the use of a mechanical ventilator) include those with diagnoses specific to lung disease, diagnoses impacting respiratory musculature, and/or diagnoses impacting structure and function of the respiratory tract/upper airway obstruction. Examples of patient diagnoses include the following:
- airway obstruction or edema, such as
- craniofacial (e.g., micrognathia in Pierre Robin sequence);
- severe subglottic stenosis;
- malacia; and
- head and neck tumor, including cancer resection and reconstructive surgery
- lung disease, such as
- bronchopulmonary dysplasia,
- ARDS, and
- cardiopulmonary disease
- neurologic/neuromuscular disorders, such as
- stroke,
- SCI, and
- degenerative disease (e.g., ALS)
- complications with premature birth and/or low birth weight, such as
- neonatal respiratory distress syndrome
Tracheostomy
Among patient populations requiring a tracheostomy, the reasons for surgical intervention (i.e., tracheotomy) or bedside percutaneous placement are varied, and the type of tracheostomy tube selected by the surgeon is individualized.
Indications for Tracheostomy
The placement of a tracheostomy tube may address the following:
- need for short- or long-term mechanical ventilation
- anatomical differences (e.g., craniofacial abnormality)
- poor secretion management—tracheostomy tube allows for suctioning of secretions in the trachea
- prolonged endotracheal intubation—which may result in laryngeal injury, patient discomfort, and problems with communication and swallowing
- upper airway obstruction such as tracheal stenosis, bilateral true vocal fold paralysis, or the presence of a mass
- need for establishing an alternative airway in the setting of failed endotracheal intubation attempts
Tracheostomy Tubes
The type of tracheostomy tube placed by the surgeon will depend on the specific needs, characteristics, anatomical variations, and medical status of the individual patient. Consideration is taken regarding the advantages offered and/or disadvantages imposed by each type of tube and its components.
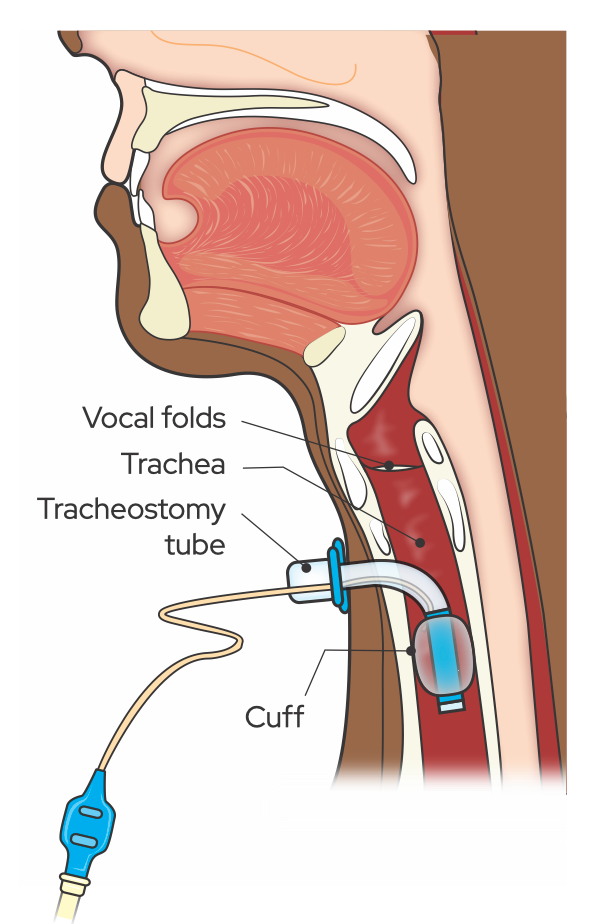
Figure 1. A tracheostomy tube in situ. From Communication and Swallowing Management of Tracheostomized and Ventilator-Dependent Individuals (3rd ed., p. 39), by K. J. Dikeman and M. S. Kazandjian, 2022, Eat Speak Breathe Publishing. Copyright 2022 by authors. Reprinted with permission.
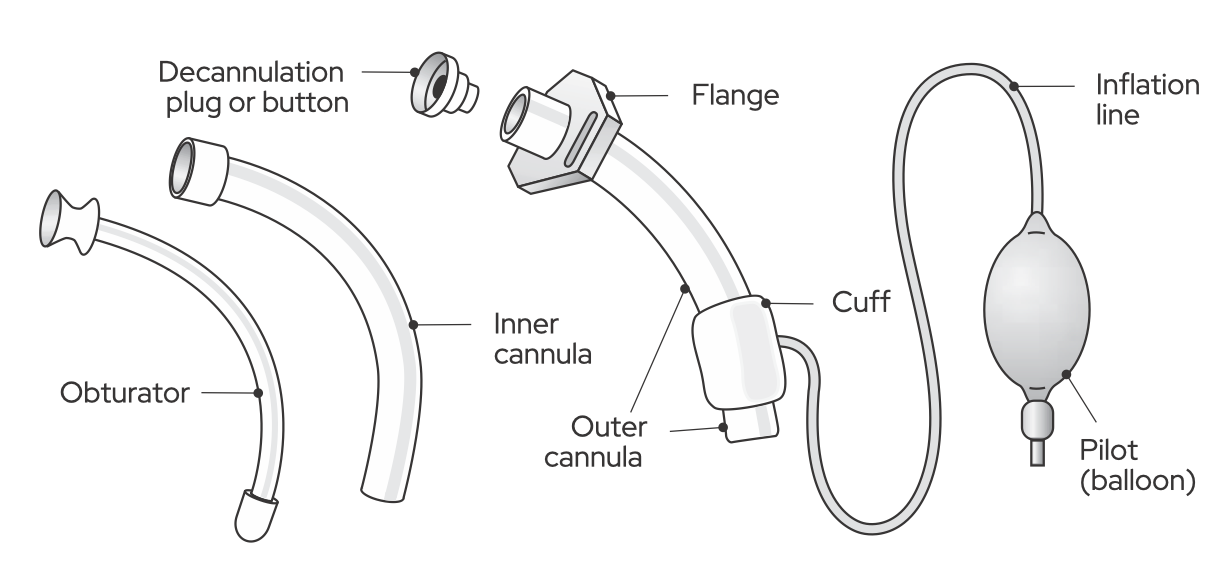
Figure 2. Parts of a standard tracheostomy tube. From Communication and Swallowing Management of Tracheostomized and Ventilator-Dependent Individuals (3rd ed., p. 58), by K. J. Dikeman and M. S. Kazandjian, 2022, Eat Speak Breathe Publishing. Copyright 2022 by authors. Reprinted with permission.
Components of Adult Tracheostomy Tubes
The components of a tracheostomy tube include the following:
- Outer cannula—the main body of the tracheostomy tube that fits directly into the tracheal stoma.
- Inner cannula—an inner tube that fits inside the outer cannula (in a dual-cannula tube), which can be disposable or reusable and may be removed to assist in cleaning and prevention of mucus plugging.
- Cannulas are measured or sized by the inner/outer diameter and length of the tube. Most tubes are sized by the measurement of the inner diameter of the outer cannula. This means that when the inner cannula is placed, the size of the tube can be 1–3 mm smaller, thus limiting the size of the airway. It is important that SLPs are familiar with sizing systems.
- Hub/connector—the external part of the tracheostomy tube cannula that allows for attachment to a mechanical ventilator or a speech valve. Most hubs are the standard 15 mm in diameter so that ventilator tubing, ventilation bags, and most one-way speaking valves will fit.
- Neck flange—a neck plate that is labeled with dimensions and type of tracheostomy tube and is attached to tracheostomy ties to secure the tube in place. Some neck flanges are fixed to the outer cannula, and others can move or swivel.
- Obturator—an instrument used during the placement of the tracheostomy tube to guide insertion into the tracheostoma and prevent tissue damage. It is removed immediately after tube placement.
- Button/cap/plug—a component used to occlude the opening of the tracheostomy tube, which may be used during the weaning and decannulation (removal of a tracheostomy tube) process and may be quickly removed if necessary.
- Cuff—an inflatable “balloon” located on the distal part of the outer cannula, which can be inflated to prevent airflow into the upper airway.
- An inflated cuff is typically used during mechanical ventilation.
- Most cuffs are inflated with air, some with water. Some tracheostomy tubes are made with foam and cannot be deflated; therefore, a one-way speaking valve should not be used with patients with a foam-filled cuff.
- Air-filled cuffs should be inflated using a manometer to measure the pressure (not to exceed 25 cmH2O). Overinflated cuffs can cut off the blood flow to the trachea and cause damage such tracheomalacia and stenosis.
- Pilot balloon—located at the distal end of an external inflation line. It indicates whether the cuff is inflated or deflated. Palpation and visual inspection of the pilot balloon cannot reliably determine the amount of air present within the cuff.
Components of Neonatal or Pediatric Tracheostomy Tubes
The components of neonatal or pediatric tracheostomy tubes are similar to those of adults, with some exceptions. Some pediatric tracheostomy tubes are cuffless, and an inflated cuff is typically used during mechanical ventilation when the pediatric patient is on high ventilator settings. Whereas most adult cuffs are inflated with air, most pediatric cuffs are inflated with water. As with adults, some tracheostomy tubes are made with foam and cannot be deflated; therefore, a one-way speaking valve should not be used with patients with a foam-filled cuff.
Types of Tracheostomy Tubes
Different types of tracheostomy tubes may include the following characteristics or specializations:
- Material variations: Tubes can be made of metal (stainless steel), plastic (polyvinyl chloride), silicone, or polyurethane.
- Disposable versus reusable.
- Single lumen (outer cannula only) versus double lumen (outer and inner cannulas).
- Cuffed versus cuffless (i.e., presence or absence of a tracheostomy tube cuff).
- Nonfenestrated (standard tube) versus fenestrated (tubes with one or more holes in the outer cannula, which, when properly seated within the airway so that the opening(s) [i.e., fenestrations] are unobstructed, allow air to pass from the trachea through the vocal folds).
- If the tracheostomy tube has an inner cannula, it must also be fenestrated or removed to take advantage of this option.
- Extended-length tracheostomy tubes (XLT), which may be proximal (i.e., an extended length from the flange to the bend) or distal (i.e., an extended length from the bend to the tube’s inferior tip).
- Talking tracheostomy tube (subglottic suction tube): a tube than runs along the side of the outer cannula with an opening on top of the cuff that not only enables the suctioning of secretions but also allows for voicing while the tracheostomy tube cuff remains inflated with the use of separate air sources for mechanical ventilation and phonation.
- FlexTend tube: a pediatric tube with a flexible tube extension intended to keep connections away from the neck, chin, and stoma.
- Specialty cannula designed to allow voicing while the tracheostomy tube cuff remains inflated (e.g., speech cannula with flaps for directing exhaled air to the upper airway).
- Tracheostomal maintenance devices (e.g., tracheostomy button, long-term cannula) may replace the tracheostomy tube as a method of maintaining the open stoma (tracheal airway).
Clinicians should properly identify/differentiate patients with tracheostomy or laryngectomy to ensure proper airway management. Total laryngectomy patients have tracheostomy tubes that, from the outside, look identical to other tubes; however, these tubes have much shorter cannulas and are not to be used in patients who have a larynx. In addition, capping and one-way speaking valve placement on a laryngectomy tube would result in airway compromise due to a lack of connection between the larynx and the nose and mouth in these patients.
The type, size, and characteristics of a patient’s tracheostomy tube may require alterations because of anatomical growth, changes in the patient’s underlying condition, and facilitation of communication options; to optimize safety; or as part of the weaning process. Note that changes made to the tracheostomy tube may impact swallow function or the ability to participate in valving trials or capping.
Tracheostomy Tube Considerations: Tube Size
The appropriate size and style of tracheostomy tubes are important when addressing communication and swallowing needs. Tracheostomy tubes may also be custom-made to fit. The tracheostomy team considers factors specific to each patient when determining the appropriate size (i.e., inner diameter, outer diameter, angle, length, etc.) of the tracheostomy tube. See Mitchell et al. (2013) and Sherman et al. (2000) for further information on patient-specific factors, including several of the following:
- Age of the patient.
- Size and anatomy of the neck and trachea (e.g., internal diameter of the trachea—a female’s trachea is approximately 3 mm smaller than a male’s).
- Communication/speech needs (e.g., tracheostomy downsize may be recommended to reduce airway resistance and increase airflow around the tracheostomy tube to enable one-way speaking valve use). The tube may need downsizing if it is too large and there is not enough leak for one-way speaking valve use.
- Indications for a tracheostomy procedure.
- Lung mechanics.
- Upper airway resistance/airway clearance.
- In pediatrics, height and weight can be used to predict the best tube size (Fenley et al., 2020).
For further information, please see Mitchell et al. (2013) and Sherman et al. (2000).
Tracheostomy Tube Considerations: Cuff Status
Consideration of the tracheostomy tube cuff status requires input from the tracheostomy team, with attention to a patient’s medical status, a patient’s respiratory/ventilatory status, and the impact it has on the patient’s communication status and swallow function.
Most cuffs can be inflated or deflated by an SLP or other medical providers (e.g., respiratory therapist, registered nurse) as necessary or when indicated (such as placing a one-way speaking valve or conducting a swallowing evaluation); however, foam cuffs cannot be inflated or deflated and may not be used with a one-way speaking valve.
Benefits of cuff deflation and cuffless tracheostomy tubes may include
- restoring airflow through the upper respiratory tract;
- the ability to use digital occlusion for phonation;
- the ability to use tracheostomy capping and/or a one-way speaking valve to allow for
- phonation or voice production both on and off of the mechanical ventilator,
- the generation of airflow through the upper airway, allowing the patient to sense secretions in order to cough, throat-clear, and/or swallow,
- improved taste and olfactory sensation as the leak allows air to move through the upper airway, and
- improved retention of moisture and humidity and screening out of microparticles;
- providing an element of safety as the patient can potentially breathe around the tracheostomy tube if there is a mucous plug;
- a reduced risk of damage to the tracheal wall caused by long-term cuff overinflation; and/or
- potential improvements in swallow physiology (Amathieu et al., 2012; D. G. Davis et al., 2002; Ding & Logemann, 2005; Hernandez et al., 2013; Mills et al., 2022; Skoretz et al., 2020).
It is important to note that an inflated cuff does not prevent aspiration as the cuff is below the level of the vocal cords.
Suctioning With Tracheostomy Tube
ASHA’s Code of Ethics stipulates that clinicians must be competent in any area in which they practice. ASHA’s Scope of Practice in Speech-Language Pathology is broad and does not address specific procedures; however, procedures should be related to the assessment and treatment of patients with communication or swallowing disorders. Some facilities (e.g., hospitals) have a process in place for “credentialing” staff in suctioning procedures and may provide training to SLPs in these procedures.
State licensure laws vary. Some states may provide specific guidance, whereas others do not. It is the clinician’s responsibility to be aware of laws and guidelines applicable to each situation. See also ASHA’s Position Statement on Multiskilled Personnel and Technical Report on Multiskilled Personnel.
Mechanical Ventilation
Mechanical ventilators are used with various modes and settings, which may require modifications as a patient’s status or condition changes. Decisions made by the physician (e.g., pulmonologist) about the mechanical ventilator modes and/or settings are carried out by trained medical professionals (e.g., respiratory therapists, nurses).
Indications for Mechanical Ventilation
Mechanical ventilation is used to address compromised breathing, which impacts the ability to move air in and out of the lungs and/or for the lungs to complete the necessary gas exchange. Respiratory or ventilatory failure (or impending failure) is an indicator of the need for mechanical ventilation. Respiratory failure may be hypoxemic (i.e., abnormally low levels of oxygen in the blood) or hypercapnic (i.e., excess carbon dioxide in the blood). Patients receive mechanical ventilation via an ETT or a tracheostomy. When a patient fails extubation (i.e., removal of the ETT), a tracheotomy may be performed to allow the patient to continue to receive mechanical ventilation.
There are many and various etiologies of respiratory failure, including, but not limited to, the following:
- lung disease (e.g., chronic obstructive pulmonary disease, pneumonia, bronchopulmonary dysplasia)
- multisystem failure or dysfunction (e.g., renal, cardiac)
- impairment or injury of the anatomical or physiological respiratory mechanism (e.g., SCI, drug overdose, chest trauma)
- neurological causes leading to nerve and muscle disorders (e.g., ALS, Guillain-Barré syndrome, stroke, Duchenne muscular dystrophy)
Mechanical ventilation may also be used with patients who undergo anesthesia (e.g., during surgery).
Mechanical Ventilator Settings/Modes
Mechanical ventilators have different settings (the characteristics of ventilation provided) and modes (representing the method—the how and when—of inspiratory support). Any changes or modifications to setting or mode are determined and managed by the physician (or the respiratory therapist/nurse/trained professional under a physician’s orders).
Mechanical Ventilator Settings
The primary mechanical ventilator settings include the following:
- Mode of ventilation.
- Tidal volume (Vt)—the size of each breath from the mechanical ventilator (measured in cubic centimeters or milliliters).
- Peak inspiratory pressure (PIP)—the highest level of pressure on inhalation.
- Rate—the number of breath cycles delivered by the mechanical ventilator per minute. The mechanical ventilator “rate” plus the patient’s spontaneous breaths equals the patient’s respiratory rate.
- Fractional inspired oxygen concentration (FiO2)—the percentage of oxygen delivered with any breath. Room air has an oxygen concentration of 21%.
- Positive end-expiratory pressure (PEEP) —the positive pressure (measured in centimeters of H2O) that is constantly applied to the airway (pressure that remains in the lungs at the end of expiration).
- Alarms—warn of possible dangers related to the patient–ventilator system, including low and high limits.
Mechanical Ventilator Modes
Mechanical ventilator modes can be either volume controlled (VC) or pressure controlled (PC), referring to which aspect(s) of the breath are preset and controlled by the ventilator. Commonly used modes of mechanical ventilation include the following:
- Assist control (AC), also known as continuous mandatory ventilation (CMV): The mechanical ventilator provides mandatory preset breaths as well as preset breaths in response to the patient’s initiation of additional breaths.
- Synchronized intermittent mandatory ventilation (SIMV): The mechanical ventilator provides mandatory preset breaths but allows the patient to breathe spontaneously between these breaths.
- Pressure support ventilation (PSV): A setting where the mechanical ventilator provides positive airway pressure with each patient-initiated breath to help them overcome the resistance of tubing and to boost tidal volume. This setting terminates positive airway pressure at the end of inspiration (i.e., augments spontaneous breathing).
- Continuous positive airway pressure (CPAP): The mechanical ventilator provides continuous positive pressure throughout the patient’s spontaneous respiratory cycle and can be used while the patient is still intubated or has an indwelling tracheostomy tube (e.g., during weaning) or noninvasively (e.g., via mask).
- Pressure-regulated volume control (PRVC): In this setting, each volume of breath that is delivered by the mechanical ventilator is based upon the pressure of the preceding breath. The cuff must remain inflated when using this mode.
Cuff Status With Mechanical Ventilation
A mechanical ventilator may be used when the tracheostomy tube cuff is inflated or deflated; however, in most cases, the cuff will remain inflated.
Possible benefits of an inflated cuff with mechanical ventilation (Dikeman & Kazandjian, 2022) include the following:
- Easier-to-maintain tidal volume and oxygen saturation because air/oxygen is not escaping through the upper airway.
- The lungs receive optimal volume and pressure delivered by the mechanical ventilator (as the inflated cuff reduces the leak so air cannot escape through the upper airway).
- Of note, cuff inflation does not prevent aspiration because aspiration happens above the level of the cuff at the vocal folds.
Impact of Tracheostomy and Ventilator Dependence on Swallowing and Communication
Placement and maintenance of an artificial airway may lead to laryngeal injury. Such injuries are often mild but may lead to persistent airway abnormalities, dysphonia, and dysphagia (Brodsky et al., 2021; Kelly et al., 2023).
Medically complex patients may have multifactorial causes of communication and swallowing problems.
- Consequences of tracheostomy and mechanical ventilator dependence on communication and psychosocial well-being may include
- aphonia/dysphonia (e.g., airflow not passing through the vocal folds);
- emotional or psychological responses (e.g., frustration, withdrawal);
- the inability to communicate wants/needs to staff and caregivers, which may impact patient safety, confidence, and psychological responses;
- the inability to hear an infant crying;
- the need for restraints in some populations (e.g., cognitively impaired patients at risk of self-decannulation);
- overall decreased quality of life and social stigma (Newman et al., 2022);
- pain/discomfort associated with communication attempts;
- reduced mobility (e.g., reduced play in children, resulting in limited opportunities for neurodevelopment);
- speech and language development in children (Fiske, 2004); and
- social skills and behavior development in children.
- Consequences of tracheostomy and mechanical ventilator dependence on swallow function may include
- disrupted breathing pattern and coordination with swallow;
- increased risk of dysphagia and aspiration (Cameron et al., 1973; L. A. Davis & Stanton, 2004; Elpern et al., 1994; Leder, 2002; Luu et al., 2022; Tolep et al., 1996);
- laryngeal/pharyngeal atrophy due to lack of airflow and disuse;
- the need for alternative forms of nutrition and hydration to maintain proper nutritional status;
- reduced/absent cough response and subglottic pressure (e.g., for expectoration of secretions);
- reduced laryngeal sensitivity;
- reduced secretion management;
- reduced senses of smell and taste;
- overall reduced quality of life, psychological impacts, and social stigma and withdrawal (Nakarada-Kordic et al., 2018); and
- an impact on oral motor and swallowing development in infants and children (Norman et al., 2007; Sillers et al., 2019).
Issues in Swallowing With Tracheostomy
Dysphagia is associated with tracheostomy placement (L. A. Davis & Stanton, 2004; Elpern et al., 1994; Leder, 2002; Tolep et al., 1996) with or without mechanical ventilation (Skoretz et al., 2020). Tracheostomy and/or intubation may impact airway patency and function by damaging or impairing the larynx (Wallace & McGrath, 2021). As such, patients with tracheostomy, especially those with uncapped tracheostomy, may be at increased risk for silent aspiration (Marvin & Thibeault, 2021).
Instrumental swallowing evaluations such as the Fiberoptic/Flexible Endoscopic Evaluation of Swallowing (FEES) and the Videofluoroscopic Swallow Study (VFSS) are the only reliable method to detect silent aspiration and to predict some risk factors for aspiration (e.g., pharyngeal residue).
The assessment and treatment of dysphagia in patients with a tracheostomy (with or without mechanical ventilator support) involves special considerations; however, the processes, procedures, approaches, and techniques used by the clinician are comparable to those used with other populations. See the information on assessment and treatment in the ASHA Practice Portal pages on Adult Dysphagia and Pediatric Feeding and Swallowing as well as the summaries of available research in the Dysphagia (Adults) Evidence Map and the Pediatric Feeding and Swallowing Evidence Map for more detailed information.
Screening for Dysphagia
The goal of dysphagia screening for patients with a tracheostomy (with or without mechanical ventilation) is to identify key factors that can help determine a patient’s readiness for clinical and/or instrumental evaluations such as level of arousal, oral motor skills, secretion management, and volitional swallow and cough ability. Screening may be conducted by an SLP or members of other trained professions.
The modified Evans blue dye test is occasionally administered at bedside on tracheostomy patients to detect potential aspiration; however, it should be conducted with caution due to inconsistent reports of its diagnostic accuracy (Béchet et al., 2016).
Clinical Swallowing Assessment
A clinical swallowing assessment may be useful in identifying signs and symptoms of feeding and swallowing difficulty and in determining a patient’s readiness for instrumental assessment. It is not necessary for a patient to be weaned from the mechanical ventilator or decannulated to begin the swallowing assessment process.
SLPs consider many details that may impact a patient’s ability to reliably participate in swallowing or feeding assessment or that may influence the assessment plan. Such considerations may include the patient’s
- Current mode of communication.
- Current level of cooperation.
- Positioning status and precautions.
- Ability to tolerate digital occlusion, a one-way speaking valve, or capping trials.
- Current medical status, including
- medical stability;
- presence of comorbidities as well as their status (i.e., temporary, permanent, degenerative, or developmental) and potential impact on swallowing function;
- frailty;
- vital signs (heart rate, oxygen saturation, respiratory rate);
- oral hygiene status and practices; and
- overall comfort, endurance, and level of fatigue.
- Contraindications to oral intake.
- Current respiratory status, including
- level of mechanical ventilator support (if any) and ventilator settings (may hold per os [PO] if too high);
- supplemental oxygen requirements (e.g., level of FiO2 and flow rate);
- oxygenation;
- frequency and type of suctioning;
- secretion levels (e.g., oral/tracheal, amount, type, consistency) and secretion management; and
- respiratory/swallowing pattern.
- Current nutritional status, including
- current mode of intake;
- current restrictions;
- whether any alternate nutrition is in place (e.g., nasogastric tube or gastrostomy tube);
- length of time without oral intake (if at all); and
- patient interest in oral intake.
- Airway history, including
- prior intubation;
- duration of intubation prior to tracheostomy; and
- prior trach placement.
- Estimated time frame for a tracheostomy tube (with or without mechanical ventilator support) to remain in place (e.g., very short duration of 1–2 days).
- Time since a tracheotomy was performed (trauma, bleeding, and edema resulting from the procedure can be present initially).
- Size and type of the tracheostomy tube (e.g., fenestrated, extended-length).
- Cuff characteristics (as applicable)—specifically, the
- type of cuff (air-filled, water-filled, or foam-filled; foam-filled cuffs cannot be deflated);
- status of cuff (deflated, partly inflated, or fully inflated); and
- cuff pressure (high or low pressure; high or low volume).
- Tolerance of any cuff deflation.
- Cuff deflation and/or reduced cuff pressure may positively impact swallow function—as cuff pressure increases, the swallowing reflex and motor movement are both suppressed (Amathieu et al., 2012; D. G. Davis et al., 2002; Ding & Logemann, 2005).
- Tolerance, when off mechanical ventilation, of tracheal occlusion (i.e., capping, one-way speaking valve, or digital).
- Use of, or candidacy for, a one-way speaking valve.
- One-way speaking valve use may positively impact swallow function in some patients (Marvin & Thibeault, 2021; O’Connor et al., 2018).
- Ability to phonate—and, if so, their vocal quality and intensity.
- Ability to perform spontaneous or elicited throat clears, coughs, and swallows to clear or expectorate secretions for the purpose of airway protection.
- Pain level with swallow.
- Emotional response (new interventions may elicit anxiety and fear).
- Goals—as well as those of their care partner.
- Ventilator status when eating (e.g., during the weaning trial)—will they be on or off the mechanical ventilator when they eat?
- Cuff status when eating (i.e., inflated or deflated).
Instrumental Swallowing Assessment
Clinicians use instrumental swallowing assessments to more comprehensively assess the physiology/pathophysiology of the patient’s swallow and the effectiveness of potential treatment strategies. FEES and VFSS may detect the presence or absence of aspiration, silent aspiration, and/or residue as well as the patient’s response to any residue and/or aspiration. When possible, completing instrumental evaluations under a variety of conditions (i.e., with the cuff inflated or deflated, on or off the mechanical ventilator, with or without a one-way speaking valve) can inform recommendations regarding multiple conditions in which a person may be able to eat and drink. Reassessment may be warranted on an ongoing basis, based on the results of treatment and goals of care.
Swallowing Treatment
Special considerations in the treatment of dysphagia in patients with a tracheostomy (with or without ventilator dependence) include the following:
- Emphasize and assist in proper oral and tracheostomy hygiene protocols, as appropriate.
- Provide oral stimulation and nonnutritive sucking experiences (when working with the infant population).
- Consult with the patient and the care team to determine the best physical positioning for the patient during swallowing treatment.
- Monitor the patient’s health status and vitals, and provide or obtain suction as needed (e.g., with clearance or assistance from a physician, a nurse, or a respiratory therapist) during any treatment activities.
- Monitor the patient’s respiratory/pulmonary status as well as mechanical ventilation needs (if the patient is on a mechanical ventilator), and collaborate with the care team (e.g., physician, respiratory therapist, nurse) for management/adjustments as appropriate and necessary.
- For patients who tolerate cuff deflation and one-way speaking valve placement, deflate the cuff and place a one-way speaking valve during treatment to normalize airflow on exhalation, restore sensory function in the upper airway, improve postural stability, possibly reduce aspiration risk, and increase cough ability.
- Use compensatory and rehabilitative techniques for swallowing and/or implement diet modifications, as appropriate.
- Initiate early rehabilitative exercise, as indicated.
- Consider risks associated with traditional compensatory strategies or postures when used by a patient with a tracheostomy (e.g., the chin-tuck posture may create a risk of occluding the tracheostomy tube or cause pain and coughing if the tube touches the tracheal wall).
- Use direct (with the use of food or liquid) and indirect (without the use of food or liquid) treatment, as appropriate.
- Consult with physical therapists, respiratory therapists, and other specialists, as indicated (e.g., pulmonary rehabilitation, respiratory muscle strength training [RMST]).
Issues in Communication With Tracheostomy
There are specific considerations when assessing, managing, and optimizing communication for individuals with tracheostomy (with or without ventilator dependence). Depending on the patient’s diagnoses, age, needs, strengths/weaknesses, and goals, the information on assessment and treatment in the following ASHA Practice Portal pages may be helpful: Acquired Apraxia of Speech, Aphasia, Augmentative and Alternative Communication, Pediatric Traumatic Brain Injury, and Traumatic Brain Injury in Adults.
Communication Assessment
The approach to providing a communication assessment to a patient with a tracheostomy, with or without ventilator dependence, involves special considerations, including, but not limited to, the following:
- The status of the patient’s medical condition (i.e., temporary, permanent, progressive/degenerative, or developmental).
- Duration of the artificial airway placement and how this affects short- and long-term communication.
- Adjustments that can be made to the tracheostomy and mechanical ventilator to improve speech communication for ventilated patients (Hoit et al., 2003; Prigent et al., 2010).
- Impact of the following factors on the choice of the most appropriate communication method. A variety of modalities may be required to support diverse communication needs:
- level of alertness/attentiveness/responsiveness
- voicing abilities (e.g., no audible phonation, reduced quality or intensity)
- respiratory status (including respiratory endurance and breath coordination)
- vocal hygiene status
- verbal fluency and ease/difficulty of speech production
- cognitive abilities
- language abilities
- developmental abilities
- motor status
- emotional and mental well-being (e.g., anxiety, depression, agitation)
- cooperation and motivation
- The presence of comorbid speech and language disorders (e.g., aphasia).
- Any communication options needed (e.g., high-tech and low-tech, oral and nonoral).
- The patient’s vision, gross and fine motor skills, and any impairment in body structure and function related to accessing AAC, as appropriate.
- The needs and goals of the patient and the care partners.
- Communication partner skills/training.
- Language(s) spoken, including preferred language.
Communication Treatment
Communication choices for people with artificial airways may include both high- and low-tech AAC options as well as both oral and nonoral options. Several options may be appropriate for a patient, depending upon their situation. Options include the following (as appropriate for the patient’s age and developmental status):
- Nonoral communication options may include
- writing;
- texting;
- gesturing and/or using signed language;
- the use of tablet or phone apps; and
- the use of low- and/or high-tech AAC.
- Oral communication options may include
- mouthing of words with lipreading by the communication partner;
- the use of leak speech with partial or full cuff deflation (using air leaking around the tracheostomy tube and passing over the vocal folds);
- the use of an electrolarynx;
- the use of digital occlusion, capping, or plugging the tracheostoma (with an uncuffed or a cuff-deflated tracheostomy tube);
- the use of a talking tracheostomy tube; and
- the use of a one-way speaking valve.
For further information, please see Ten Hoorn et al. (2016), Rose et al. (2021), and Wallace et al. (2023).
Verbal communication options may require modification to the current tracheostomy tube (or a change in the type of tube), cuff inflation status, and/or mechanical ventilator settings. These changes involve input from the tracheostomy team members (e.g., SLP, registered nurse, respiratory therapist, physician). It can be helpful for a patient with a tracheostomy tube to have a nonoral backup system of communication to meet diverse communication needs of communication partners and across contexts.
One-Way Speaking Valves
One-way speaking valves enable individuals with a tracheostomy tube to use voice and speech to communicate. A variety of one-way speaking valves are available, and each speaking valve may look and work somewhat differently from the others. In general, one-way speaking valves open on inhalation and close on expiration to redirect expired air through the upper airway and vocal folds. Some one-way speaking valves can be placed in line with a mechanical ventilator. Early speech intervention and voice restoration for a patient with mechanical ventilation may lead to increased participation in their care and improved quality of life (Freeman-Sanderson et al., 2016). Candidacy for speaking valve use must be carefully established.
Candidacy requirements for safe and effective one-way speaking valve placement and use include the following (Hess & Altobelli, 2014):
- Must be awake, alert, and attempting to communicate.
- Must be medically stable.
- Must tolerate cuff deflation (if using a cuffed tracheostomy tube).
- Must be able to exhale around the tracheostomy tube and through the upper airway.
- Manometry can be used to measure transtracheal pressure to determine airway patency. The manometer is placed between the patient and the trach hub for patients off the mechanical ventilator and in line with the ventilator circuit for patients on mechanical ventilation. Transtracheal pressure is the number at the end of the exhalation.
Candidacy considerations may also include the following (Hess & Altobelli, 2014):
- The presence of back pressure signs or poor tolerance may indicate candidacy limitations.
- The possible requirement of a tracheostomy tube downsize or a change in the type of tracheostomy tube.
- The possible requirement of changes in mechanical ventilator settings.
- Anxiety or discomfort associated with one-way speaking valve placement and a redirection of airflow.
- Behaviors associated with one-way speaking valve placement may be physiological and/or behavioral in individuals who are unable to reliably communicate (e.g., infants and young children). These signs can be misunderstood by some clinicians; therefore, the use of manometry to objectively measure transtracheal pressure may more accurately determine success.
Benefits of one-way speaking valve use may include the following:
- improved vocal quality, intensity, fluidity, length of utterances, and duration of phonation during connected speech
- improved cough and secretion management
- increased upper airway sensation, including olfaction
- possible benefits to swallowing function (which can be identified during the swallowing assessment)
- optimizing communication
- improved quality of life and self-advocacy
- expedited weaning and decannulation process
For further information, see Wallace et al. (2023).
Prior to one-way speaking valve placement, the airway should be cleared of secretions, and the cuff should be deflated. If the patent displays signs of respiratory distress (e.g., increased respiratory rate, diminished oxygen saturation, increased heart rate) following placement of the speaking valve, then the one-way speaking valve should be removed immediately, and the team should assess airway patency and evaluate for possible causes of difficulty (Hess & Altobelli, 2014; Sutt et al., 2021).
Contraindications for one-way speaking valve use include the following:
- unconscious patients
- foam cuff tracheostomy tube
- inflated tracheostomy tube cuff
- significant airway obstruction (e.g., subglottic stenosis), which may prevent sufficient exhalation
- laryngectomy
- laryngotracheal separation
- unmanageable copious and/or thick secretions
- high mechanical ventilator settings (e.g., positive end-expiratory pressure [PEEP], fractional inspired oxygen concentration [FiO2], or peak inspiratory pressure [PIP] settings)
- medical instability
Considerations for Pediatric Populations
Infants and children have different airway anatomy, physiology, and size than adults (Singh & Zubair, 2023). Due to these differences, the typical surgical incision for pediatric patients is vertical through the third and fourth tracheal rings, rather than the horizontal incision typically used in adults between the second and third tracheal rings. Pediatric tracheostomy tubes and mechanical ventilator settings may differ from those typically used with adults. For instance, manufacturers produce smaller tracheostomy tubes specifically for use with pediatric and neonatal populations. These tubes can be cuffed or cuffless. Additionally, in contrast to adult populations, pediatric tracheostomy tubes are often single lumen, with no removable inner cannula. SLPs should also consider anatomical and physiological differences between pediatric and adult populations when conducting a swallow evaluation. Premature infants who are status post prolonged intubation may have limited per os (PO) experience, and the suck–swallow–breathe sequence in infants on mechanical ventilation may be discoordinated.
Uncuffed/cuffless tracheostomy tubes are generally preferred over cuffed tracheostomy tubes in the pediatric population; however, much like adult patients, cuffed tracheostomy tubes may be necessary in certain patient populations with comorbidities such as secretion management difficulties, like those with severe pulmonary disease or neuromuscular disease.
The mechanical ventilator modes used for pediatric patients may also differ significantly from those used with adults. There are a variety of mechanical ventilator modes available for neonatal and pediatric use, each with benefits and drawbacks but with no definitive indications or protocols for use (Kollisch-Singule et al., 2021).
SLPs should also be aware that reasons for tracheostomy may differ between adults and infants. For instance, infants often need tracheostomy tube placement due to complications from prematurity (chronic lung disease) or aerodigestive conditions (subglottic stenosis, other laryngeal abnormalities). Primary indicators for pediatric tracheostomy may also include prematurity, cardiopulmonary disease, neurological impairment, airway obstruction, craniofacial abnormalities (e.g., Treacher Collins syndrome, Pierre Robin sequence), and traumatic injury (Gergin et al., 2016). For further information, please see Singh and Zubair (2023).
Clinicians may rely on nonverbal signs to detect pain and discomfort (e.g., cries, facial expressions, unusual postures, agitated movements) when limited communication is present. A delay in remediating pain or discomfort can result in instability in heart rate, respiration, tone, or other physiologic function. SLPs may provide education to family members about interventions to reduce pain and discomfort (e.g., swaddling, nonnutritive sucking).
Tracheostomy tube placement and mechanical ventilator dependence can impact social development in pediatric populations. SLPs may need to make modifications to the environment, patient positioning, and patient handling to improve social interactions. Physical therapy and occupational therapy may be consulted as necessary. SLPs provide counseling and education to family members regarding the developmental status of communication, swallowing, voice, articulation, and related functions as appropriate.
As with all patients, holistic, interprofessional, and patient/family-centered care is important to achieving the best treatment outcome.
ASHA Resources
- Aerosol Generating Procedures
- Code of Ethics
- Flexible Endoscopic Evaluation of Swallowing (FEES)
- International Classification of Functioning, Disability, and Health (ICF)
- Interprofessional Education/Interprofessional Practice (IPE/IPP)
- Position Statement: Multiskilled Personnel
- Scope of Practice in Speech-Language Pathology
- Social Determinants of Health
- Special Interest Group 13, Swallowing and Swallowing Disorders (Dysphagia)
- Technical Report: Multiskilled Personnel
- Videofluoroscopic Swallow Study (VFSS)
Other Resources
This list of resources is not exhaustive, and the inclusion of any specific resource does not imply endorsement from ASHA.
- American Academy of Otolaryngology-Head and Neck Surgery
- American Thoracic Society
- Clinical Utility and Future Direction of Speaking Valve: A Review
- International Ventilator Users Network
- Passy Muir
- RCSLT: New Long COVID Guidance and Patient Handbook
- The Global Tracheostomy Collaborative
- United Kingdom National Tracheostomy Safety Project
Abe, T., Madotto, F., Pham, T., Nagata, I., Uchida, M., Tamiya, N., Kurahashi, K., Bellani, G., Laffey, J. G., & the LUNG-SAFE Investigators and the ESICM Trials Group. (2018). Epidemiology and patterns of tracheostomy practice in patients with acute respiratory distress syndrome in ICUs across 50 countries. Critical Care, 22(1), Article 195. https://doi.org/10.1186/s13054-018-2126-6
Abril, M. K., Berkowitz, D. M., Chen, Y., Waller, L. A., Martin, G. S., & Kempker, J. A. (2021). The epidemiology of adult tracheostomy in the United States 2002–2017: A serial cross-sectional study. Critical Care Explorations, 3(9), e0523. https://doi.org/10.1097/CCE.0000000000000523
Amathieu, R., Sauvat, S., Reynaud, P., Slavov, V., Luis, D., Dinca, A., Tual, L., Bloc, S., & Dhonneur, G. (2012). Influence of the cuff pressure on the swallowing reflex in tracheostomized intensive care unit patients. British Journal of Anaesthesia, 109(4), 578–583. https://doi.org/10.1093/bja/aes210
American Speech-Language-Hearing Association. (2016). Scope of practice in speech-language pathology [Scope of practice]. https://www.asha.org/policy/
American Speech-Language-Hearing Association. (2023). Code of ethics [Ethics]. https://www.asha.org/policy/
Béchet, S., Hill, F., Gilheaney, Ó., & Walshe, M. (2016). Diagnostic accuracy of the modified Evan’s blue dye test in detecting aspiration in patients with tracheostomy: A systematic review of the evidence. Dysphagia, 31(6), 721–729. https://doi.org/10.1007/s00455-016-9737-3
Bonvento, B., Wallace, S., Lynch, J., Coe, B., & McGrath, B. A. (2017). Role of the multidisciplinary team in the care of the tracheostomy patient. Journal of Multidisciplinary Healthcare, 10, 391–398. https://doi.org/10.2147/JMDH.S118419
Cameron, J. L., Reynolds, J., & Zuidema, G. D. (1973). Aspiration in patients with tracheostomies. Surgery, Gynecology & Obstetrics, 136, 68–70.
Brenner, M. J., Pandian, V., Milliren, C. E., Graham, D. A., Zaga, C., Morris, L. L., Bedwell, J. R., Das, P., Zhu, H., Allen, J. L. Y., Peltz, A., Chin, K.,Schiff, B. A., Randall, D. M., Swords, C., French, D., Ward, E., Sweeney, J. M., Warrillow, S. J., . . . Roberson, D. W. (2020). Global Tracheostomy Collaborative: Data-driven improvements in patient safety through multidisciplinary teamwork, standardisation, education, and patient partnership. British Journal of Anaesthesia, 125(1), e104–e118. https://doi.org/10.1016/j.bja.2020.04.054
Brodsky, M. B., Akst, L. M., Jedlanek, E., Pandian, V., Blackford, B., Price, C., Cole, G., Mendez-Tellez, P. A., Hillel, A. T., Best, S. R., & Levy, M. J. (2021). Laryngeal injury and upper airway symptoms after endotracheal intubation during surgery: A systematic review and meta-analysis. Anesthesia and Analgesia, 132(4), 1023–1032. https://doi.org/10.1213/ANE.0000000000005276
Chatterjee, A., Chen, M., Gialdini, G., Reznik, M. E., Murthy, S., Kamel, H., & Merkler, A. E. (2018). Trends in tracheostomy after stroke: Analysis of the 1994 to 2013 National Inpatient Sample. The Neurohospitalist, 8(4), 171–176. https://doi.org/10.1177/1941874418764815
Chorney, S. R., Brown, A. F., Brooks, R. L., Bailey, C., Whitney, C., Sewell, A., & Johnson, R. F. (2021). Pediatric tracheostomy outcomes after development of a multidisciplinary airway team: A quality improvement initiative. OTO Open, 5(3), Article 2473974X211045615. https://doi.org/10.1177/2473974X211045615
COVIDSurg Collaborative. (2021). Head and neck cancer surgery during the COVID-19 pandemic: An international, multicenter, observational cohort study. Cancer, 127(14), 2476–2488. https://doi.org/10.1002/cncr.33320
Davis, D. G., Bears, S., Barone, J. E., Corvo, P. R., & Tucker, J. B. (2002). Swallowing with a tracheostomy tube in place: Does cuff inflation matter? Journal of Intensive Care Medicine, 17(3), 132–135. https://doi.org/10.1177/088506660201700304
Davis, L. A., & Stanton, S. T. (2004). Characteristics of dysphagia in elderly patients requiring mechanical ventilation. Dysphagia, 19, 7–14. https://doi.org/10.1007/s00455-003-0017-7
Davis, S. D., Weyh, A. M., Salman, S. O., Madbak, F., & Fraker, J. T. (2021). Speech pathology services are integral, but underutilized in tracheostomy rehabilitation. Craniomaxillofacial Trauma & Reconstruction, 14(2), 110–118. https://doi.org/10.1177/1943387520948381
Dikeman, K. J., & Kazandjian, M. S. (2022). Communication and swallowing management of tracheostomized and ventilator-dependent individuals (3rd ed.). Eat Speak Breathe Publishing.
Ding, R., & Logemann, J. A. (2005). Swallow physiology in patients with trach cuff inflated or deflated: A retrospective study. Head & Neck: Journal of the Sciences and Specialties of the Head and Neck, 27(9), 809–813. https://doi.org/10.1002/hed.20248
Elpern, E. H., Scott, M. G., Petro, L., & Ries, M. H. (1994). Pulmonary aspiration in mechanically ventilated patients with tracheostomies. Chest, 105(2), 563–566. https://doi.org/10.1378/chest.105.2.563
Fenley, H., Voorman, M., Dove, J. T., & Greene, J. S. (2020). Predicting pediatric tracheal airway size from anthropomorphic measurements. International Journal of Pediatric Otorhinolaryngology, 134, Article 110020. https://doi.org/10.1016/j.ijporl.2020.110020
Fiske, E. (2004). Effective strategies to prepare infants and families for home tracheostomy care. Advances in Neonatal Care, 4(1), 42–53. https://doi.org/10.1016/j.adnc.2003.11.011
Freeman-Sanderson, A., Togher, L., Phipps, P., & Elkins, M. (2011). A clinical audit of the management of patients with a tracheostomy in an Australian tertiary hospital intensive care unit: Focus on speech-language pathology. International Journal of Speech-Language Pathology, 13(6), 518–525. https://doi.org/10.3109/17549507.2011.582520
Freeman-Sanderson, A., Togher, L., Elkins, M., & Phipps, P. (2016). An intervention to allow early speech in ventilated tracheostomy patients in an Australian intensive care unit (ICU): A randomised controlled trial. Australian Critical Care, 29(2), 114. https://doi.org/10.1016/j.aucc.2015.12.012
Gergin, O., Adil, E. A., Kawai, K., Watters, K., Moritz, E., & Rahbar, R. (2016). Indications of pediatric tracheostomy over the last 30 years: Has anything changed? International Journal of Pediatric Otorhinolaryngology, 87, 144–147. https://doi.org/10.1016/j.ijporl.2016.06.018
Hernandez, G., Pedrosa, A., Ortiz, R., Accuaroni, M. D. M. C., Cuena, R., Collado, C. V., Plaza, S. G., Arenas, P. G., & Fernandez, R. (2013). The effects of increasing effective airway diameter on weaning from mechanical ventilation in tracheostomized patients: A randomized controlled trial. Intensive Care Medicine, 39(6), 1063–1070. https://doi.org/10.1007/s00134-013-2870-7
Hess, D. R., & Altobelli, N. P. (2014). Tracheostomy tubes. Respiratory Care, 59(6), 956–973. https://doi.org/10.4187/respcare.02920
Hoit, J. D., Banzett, R. B., Lohmeier, H. L., Hixon, T. J., & Brown, R. (2003). Clinical ventilator adjustments that improve speech. Chest, 124(4), 1512–1521. https://doi.org/10.1378/chest.124.4.1512
Janik, S., Grasl, S., Yildiz, E., Besser, G., Kliman, J., Hacker, P., Frommlet, F., Fochtmann-Frana, A., & Erovic, B. M. (2021). A new nomogram to predict the need for tracheostomy in burned patients. European Archives of Oto-Rhino-Laryngology, 278(9), 3479–3488. https://doi.org/10.1007/s00405-020-06541-3
Kelly, E., Hirschwald, J., Clemens, J., & Regan, J. (2023). Persistent features of laryngeal injury following endotracheal intubation: A systematic review. Dysphagia, 38(5), 1333–1341. https://doi.org/10.1007/s00455-023-10559-0
Kollisch-Singule, M., Ramcharran, H., Satalin, J., Blair, S., Gatto, L. A., Andrews, P. L., Habashi, N. M., Nieman, G. F., & Bougatef, A. (2021). Mechanical ventilation in pediatric and neonatal patients. Frontiers in Physiology, 12, Article 805620. https://doi.org/10.3389/fphys.2021.805620
Leder, S. B. (2002). Incidence and type of aspiration in acute care patients requiring mechanical ventilation via a new tracheotomy. Chest, 122(5), 1721–1726. https://doi.org/10.1378/chest.122.5.1721
Lone, N. I., & Walsh, T. S. (2011). Prolonged mechanical ventilation in critically ill patients: Epidemiology, outcomes and modelling the potential cost consequences of establishing a regional weaning unit. Critical Care, 15(2), Article R102. https://doi.org/10.1186/cc10117
Long, P., Sun, D., & Zhang, Z. (2022). Risk factors for tracheostomy after traumatic cervical spinal cord injury: A 10-year study of 456 patients. Orthopaedic Surgery, 14(1), 10–17. https://doi.org/10.1111/os.13172
Luu, K., Belsky, M. A., Dharmarajan, H., Kaffenberger, T., McCoy, J. L., Cangilla, K., Tobey, A. B. J., Simons, J. P., Maguire, R., & Padia, R. (2022). Dysphagia in pediatric patients with tracheostomy. Annals of Otology, Rhinology & Laryngology, 131(5), 457–462. https://doi.org/10.1177/00034894211025179
Mahmood, K., Cheng, G. Z., Van Nostrand, K., Shojaee, S., Wayne, M. T., Abbott, M., Nettlow, D., Parish, A., Green, C. L., Safi, J., Brenner, M. J., & De Cardenas, J. (2021). Tracheostomy for COVID-19 respiratory failure: Multidisciplinary, multicenter data on timing, technique, and outcomes. Annals of Surgery, 274(2), 234–239. https://doi.org/10.1097/SLA.0000000000004955
Martin-Villares, C., Perez Molina-Ramirez, C., Bartolome-Benito, M., Bernal-Sprekelsen, M., & COVID ORL ESP Collaborative Group. (2021). Outcome of 1890 tracheostomies for critical COVID-19 patients: A national cohort study in Spain. European Archives of Oto-Rhino-Laryngology, 278(5), 1605–1612. https://doi.org/10.1007/s00405-020-06220-3
Marvin, S., & Thibeault, S. L. (2021). Predictors of aspiration and silent aspiration in patients with new tracheostomy. American Journal of Speech-Language Pathology, 30(6), 2554–2560. https://doi.org/10.1044/2021_AJSLP-20-00377
Mehta, A. B., Syeda, S. N., Bajpayee, L., Cooke, C. R., Walkey, A. J., & Wiener, R. S. (2015). Trends in tracheostomy for mechanically ventilated patients in the United States, 1993–2012. American Journal of Respiratory and Critical Care Medicine, 192(4), 446–454. https://doi.org/10.1164/rccm.201502-0239OC
Mills, C. S., Michou, E., King, N., Bellamy, M. C., Siddle, H. J., Brennan, C. A., & Bojke, C. (2022). Evidence for above cuff vocalization in patients with a tracheostomy: A systematic review. The Laryngoscope, 132(3), 600–611. https://doi.org/10.1002/lary.29591
Mitchell, R. B., Hussey, H. M., Setzen, G., Jacobs, I. N., Nussenbaum, B., Dawson, C., Brown, C. A., III, Brandt, C., Deakins, K., Hartnick, C., & Merati, A. (2013). Clinical consensus statement: Tracheostomy care. Otolaryngology–Head and Neck Surgery, 148(1), 6–20. https://doi.org/10.1177/0194599812460376
Mourelo, M., Galeiras, R., Pértega, S., Freire, D., López, E., Broullón, J., & Campos, E. (2015). Tracheostomy in the management of patients with thermal injuries. Indian Journal of Critical Care Medicine, 19(8), 449–455. https://doi.org/10.4103/0972-5229.162460 [PDF]
Mu, Z., & Zhang, Z. (2019). Risk factors for tracheostomy after traumatic cervical spinal cord injury. Journal of Orthopaedic Surgery, 27(3). https://doi.org/10.1177/2309499019861809
Muller, R. G., Mamidala, M. P., Smith, S. H., Smith, A., & Sheyn, A. (2019). Incidence, epidemiology, and outcomes of pediatric tracheostomy in the United States from 2000 to 2012. Otolaryngology–Head and Neck Surgery, 160(2), 332–338. https://doi.org/10.1177/0194599818803598
Nakarada-Kordic, I., Patterson, N., Wrapson, J., & Reay, S. D. (2018). A systematic review of patient and caregiver experiences with a tracheostomy. The Patient: Patient-Centered Outcomes Research, 11(2), 175–191. https://doi.org/10.1007/s40271-017-0277-1
Newman, H., Clunie, G., Wallace, S., Smith, C., Martin, D., & Pattison, N. (2022). What matters most to adults with a tracheostomy in ICU and the implications for clinical practice: A qualitative systematic review and metasynthesis. Journal of Critical Care, 72, Article 154145. https://doi.org/10.1016/j.jcrc.2022.154145
Ninan, A., Grubb, L. M., Brenner, M. J., & Pandian, V. (2023). Effectiveness of interprofessional tracheostomy teams: A systematic review. Journal of Clinical Nursing, 32(19–20), 6967–6986. https://doi.org/10.1111/jocn.16815
Norman, V., Louw, B., & Kritzinger, A. (2007). Incidence and description of dysphagia in infants and toddlers with tracheostomies: A retrospective review. International Journal of Pediatric Otorhinolaryngology, 71(7), 1087–1092. https://doi.org/10.1016/j.ijporl.2007.03.018
O’Connor, L. R., Morris, N. R., & Paratz, J. (2018). Physiological and clinical outcomes associated with use of one-way speaking valves on tracheostomised patients: A systematic review. Heart & Lung, 48(4), 356–364. https://doi.org/10.1016/j.hrtlng.2018.11.006
Pelosi, P., Ferguson, N. D., Frutos-Vivar, F., Anzueto, A., Putensen, C., Raymondos, K., Apezteguia, C., Desmery, P., Hurtado, J., Abroug, F., Elizalde, J., Tomicic, V., Cakar, N., Gonzalez, M., Arabi, Y., Moreno, R., Esteban, A., & the Ventila Study Group. (2011). Management and outcome of mechanically ventilated neurologic patients. Critical Care Medicine, 39(6), 1482–1492. https://doi.org/10.1097/CCM.0b013e31821209a8
Prigent, H., Garguilo, M., Pascal, S., Pouplin, S., Bouteille, J., Lejaille, M., Orlikowski, D., & Lofaso, F. (2010). Speech effects of a speaking valve versus external PEEP in tracheostomized ventilator-dependent neuromuscular patients. Intensive Care Medicine, 36(10), 1681–1687. https://doi.org/10.1007/s00134-010-1935-0
Robba, C., Galimberti, S., Graziano, F., Wiegers, E. J. A., Lingsma, H. F., Iaquaniello, C., Stocchetti, N., Menon, D., Citerio, G., & The CENTER-TBI ICU Participants and Investigators. (2020). Tracheostomy practice and timing in traumatic brain-injured patients: A CENTER-TBI study. Intensive Care Medicine, 46(5), 983–994. https://doi.org/10.1007/s00134-020-05935-5
Rose, L., Sutt, A.-L., Amaral, A. C., Fergusson, D. A., Smith, O. M., & Dale, C. M. (2021). Interventions to enable communication for adult patients requiring an artificial airway with or without mechanical ventilator support. Cochrane Database of Systematic Reviews. https://doi.org/10.1002/14651858.CD013379.pub2
Segura, T., Medrano, I. H., Collazo, S., Maté, C., Sguera, C., Del Rio-Bermudez, C., Casero, H., Salcedo, I., García-García, J., Alcahut-Rodríguez, C., Savana Research Group, & Taberna, M. (2023). Symptoms timeline and outcomes in amyotrophic lateral sclerosis using artificial intelligence. Scientific Reports, 13(1), Article 702. https://doi.org/10.1038/s41598-023-27863-2
Sherman, J. M., Davis, S., Albamonte-Petrick, S., Chatburn, R. L., Fitton, C., Green, C., Johnston, J., Lyrene, R. K., Myer, C., 3rd, Othersen, H. B., Wood, R., Zach, M., Zander, J., & Zinman, R. (2000). Care of the child with a chronic tracheostomy. American Journal of Respiratory and Critical Care Medicine, 161(1), 297–308. https://doi.org/10.1164/ajrccm.161.1.ats1-00
Siddiqui, A. S., Dogar, S. A., Lal, S., Akhtar, S., & Khan, F. A. (2016). Airway management and postoperative length of hospital stay in patients undergoing head and neck cancer surgery. Journal of Anaesthesiology Clinical Pharmacology, 32(1), 49–53. https://doi.org/10.4103/0970-9185.173341
Sillers, L., DeMauro, S., Lioy, J., & Moran, K. (2019). Feeding outcomes following infant tracheostomy. Pediatrics, 144(2), 480. https://doi.org/10.1542/peds.144.2MA5.480
Singh, A., & Zubair, A. (2023). Pediatric tracheostomy [Continuing Medical Education Online Learning Module]. In StatPearls [Continuing Medical Education Learning Management System]. StatPearls Publishing. https://pubmed.ncbi.nlm.nih.gov/32809457/
Skoretz, S. A., Anger, N., Wellman, L., Takai, O., & Empey, A. (2020). A systematic review of tracheostomy modifications and swallowing in adults. Dysphagia, 35(6), 935–947. https://doi.org/10.1007/s00455-020-10115-0
Society of Critical Care Medicine. (n.d.). Critical care statistics. Accessed November 16, 2023, from https://www.sccm.org/Communications/Critical-Care-Statistics
Spataro, R., Bono, V., Marchese, S., & La Bella, V. (2012). Tracheostomy mechanical ventilation in patients with amyotrophic lateral sclerosis: Clinical features and survival analysis. Journal of the Neurological Sciences, 323(1–2), 66–70. https://doi.org/10.1016/j.jns.2012.08.011
Sutt, A.-L., Wallace, S., & Egbers, P. (2021). Upper airway assessment for one-way valve use in a patient with a tracheostomy. American Journal of Speech-Language Pathology, 30(6), 2716–2717. https://doi.org/10.1044/2021_AJSLP-21-00174
Ten Hoorn, S., Elbers, P. W., Girbes, A. R., & Tuinman, P. R. (2016). Communicating with conscious and mechanically ventilated critically ill patients: A systematic review. Critical Care, 20(1), Article 333. https://doi.org/10.1186/s13054-016-1483-2
Tolep, K., Getch, C. L., & Criner, G. J. (1996). Swallowing dysfunction in patients receiving prolonged mechanical ventilation. Chest, 109(1), 167–172. https://doi.org/10.1378/chest.109.1.167
Wahlster, S., Sharma, M., Chu, F., Granstein, J. H., Johnson, N. J., Longstreth, W. T., & Creutzfeldt, C. J. (2021). Outcomes after tracheostomy in patients with severe acute brain injury: A systematic review and meta-analysis. Neurocritical Care, 34(3), 956–967. https://doi.org/10.1007/s12028-020-01109-9
Walcott, B. P., Kamel, H., Castro, B., Kimberly, W. T., & Sheth, K. N. (2014). Tracheostomy after severe ischemic stroke: A population-based study. Journal of Stroke & Cerebrovascular Diseases, 23(5), 1024–1029. https://doi.org/10.1016/j.jstrokecerebrovasdis.2013.08.019
Wallace, S., McGowan, S., & Sutt, A.-L. (2023). Benefits and options for voice restoration in mechanically ventilated intensive care unit patients with a tracheostomy. Journal of the Intensive Care Society, 24(1), 104–111. https://doi.org/10.1177/17511437221113162
Wallace, S., & McGrath, B. A. (2021). Laryngeal complications after tracheal intubation and tracheostomy. The British Journal of Anaesthesia, 21(7), 250–257. https://doi.org/10.1016/j.bjae.2021.02.005
Whitmore, K. A., Townsend, S. C., & Laupland, K. B. (2020). Management of tracheostomies in the intensive care unit: A scoping review. BMJ Open Respiratory Research, 7(1), Article e000651. https://doi.org/10.1136/bmjresp-2020-000651
Acknowledgments
Content for ASHA’s Practice Portal is developed through a comprehensive process that includes multiple rounds of subject matter expert input and review. ASHA extends its gratitude to the following subject matter experts who were involved in the development of the Tracheostomy and Ventilator Dependence page:
- Suzanne Abraham, PhD, CCC-SLP
- Carmin Bartow, MS, CCC-SLP
- Laura Brooks, MEd, CCC-SLP
- Karen Dikeman, MA, CCC-SLP
- Amy Freeman-Sanderson, PhD, CPSP
- Roxann Diez Gross, PhD, CCC-SLP
- Alice Inman, MS, CCC-SLP
- Julie Kobak, MA, CCC-SLP
- Katy Peck, MA, CCC-SLP
- Donna Scarborough, PhD, CCC-SLP
- Stacey Skoretz, PhD, CCC-SLP
- Donna Tippett, MA, CCC-SLP
- Kathleen Wengel, MS, CCC-SLP
Citing Practice Portal Pages
The recommended citation for this Practice Portal page is:
American Speech-Language-Hearing Association. (n.d.). Tracheostomy and ventilator dependence [Practice portal]. https://www.asha.org/Practice-Portal/Professional-Issues/Tracheostomy-and-Ventilator-Dependence/
Content Disclaimer: The Practice Portal, ASHA policy documents, and guidelines contain information for use in all settings; however, members must consider all applicable local, state and federal requirements when applying the information in their specific work setting.












