Hearing Loss in Adults
The scope of this page is hearing loss in adult populations aged 18 years and older.
See the Hearing Loss (Adults) Evidence Map for summaries of the available research on this topic.
Hearing-related terminology may vary depending upon context and a range of factors. See the American Speech-Language-Hearing Association (ASHA) resource on hearing-related topics: terminology guidance for more information.
Hearing loss refers to a partial or total inability to hear. It can result from problems with the ear (outer, middle, and/or inner), the vestibulocochlear nerve (i.e., cranial nerve eight or CN VIII), and/or the auditory system. In the context of this page, hearing loss refers to an audiologic diagnosis of hearing thresholds outside the range of typical hearing.
Hearing loss has a variety of causes and may be
- bilateral or unilateral,
- symmetrical (degree and configuration of hearing loss are the same in each ear) or asymmetrical,
- progressive or sudden in onset,
- fluctuating or stable, and
- present at birth or acquired at some point during an individual’s life.
Hearing loss can be described by variation in type, degree, and configuration. The three basic types of hearing loss are sensorineural, conductive, and mixed.
- Sensorineural hearing loss is due to cochlear (sensory) or vestibulocochlear nerve/CN VIII (neural) auditory dysfunction.
- Conductive hearing loss is due to a problem conducting sound waves through the outer ear canal, tympanic membrane, or middle ear (ossicles).
- Mixed hearing loss is the result of damage to conductive pathways of the outer and/or middle ear and to the nerves or sensory hair cells of the inner ear.
The degree of hearing loss refers to level of severity. The degree of hearing loss can have significant implications for an individual (e.g., limiting the ability to understand speech in background noise, decreasing the enjoyment of music, impacting overall quality of life).
The table below shows one commonly used classification system.
| Degree of hearing loss | Hearing loss range (in dB HL) |
| Normal | –10 to 15 |
| Slight | 16 to 25 |
| Mild | 26 to 40 |
| Moderate | 41 to 55 |
| Moderately severe | 56 to 70 |
| Severe | 71 to 90 |
| Profound | 91+ |
| Note. dB HL = decibels in hearing level. Adapted from Clark (1981). | |
The configuration, or shape, of the hearing loss refers to the pattern of hearing loss across frequencies, as illustrated in a graph called an audiogram. For example, flat hearing loss configurations indicate approximately the same amount of hearing loss for low and high frequencies, whereas the configuration for a high-frequency or a low-frequency loss will appear sloped.
The assessment, treatment, and management of hearing loss and related disorders is often an interprofessional process. Audiologists, speech-language pathologists, otolaryngologists, primary care physicians, and various other specialists may be involved. See the ASHA resource on interprofessional education/interprofessional practice (IPE/IPP) for more information on interprofessional collaborative practice.
An individual with hearing loss and their family (which includes, for the purpose of this page, family members, significant others, caregivers, and support system members) are integral to the assessment, treatment, and management process, including planning, decision making, and service delivery. Comprehensive hearing health provision models include person- and family-centered approaches (Grenness et al., 2014; Scarinci et al., 2013). ASHA resources on this topic include person-centered care in audiology and the ASHA Practice Portal page on Cultural Responsiveness. Visit the Ida Institute and the Institute for Patient- and Family-Centered Care for more information on person-centered hearing health care.
The incidence of a disorder or condition refers to the number of new cases identified in a specified time period. Prevalence refers to the number of individuals who are living with the disorder or condition in a given time period.
As of 2018, 432 million adults worldwide demonstrated a disabling hearing loss, or a hearing loss greater than 40 decibels (dB), resulting in an overall prevalence rate of 7.6% of adults aged 15 years and older. Hearing loss was found to be more prevalent among males (8.5%) than females (6.7%; World Health Organization [WHO], 2018). Current trends indicate that this prevalence rate is increasing, with as many as one in four people projected to be living with some degree of hearing loss by 2050 (WHO, 2021a). As many as one in 10 people are estimated to have a disabling hearing loss that will require rehabilitation (WHO, 2021b).
In the United States, hearing loss is the third most common chronic physical condition (National Center for Environmental Health, 2018). According to the 2018 National Health Interview Survey, 16.5% of adults aged 18 years and older report “a little trouble hearing,” “moderate trouble,” “a lot of trouble,” or “deaf” without the use of hearing aids or other listening devices (National Center for Health Statistics, 2018). Reported hearing difficulties increase with the age of the individual: 6.1% of adults aged 18–44 years, 17.8% of adults aged 45–64 years, 31.6% of adults aged 65–74 years, and 47.2% of adults aged 75 years and older indicate hearing trouble (Villarroel et al., 2019). Studies have found that as many as 81.4% of individuals aged 80 years or older have some amount of hearing loss (Sharma et al., 2020).
- In adults who have hearing loss since birth or childhood, approximately half of all prelingual hearing loss cases are due to genetic causes, including genetic syndromes (e.g., CHARGE syndrome, Usher syndrome) and nonsyndromic genetic inheritance (e.g., autosomal dominant nonsyndromic hearing loss, X-linked nonsyndromic hearing loss; Sheffield & Smith, 2019). The other half of hearing loss cases beginning in childhood are due to preventable causes (WHO, 2021b).
- In young and middle-aged adults, excessive noise exposure is the most common, preventable cause of hearing loss (Carroll et al., 2017). Occupational noise-induced hearing loss is the most common occupational disease in the world (Chen et al., 2020; Lie et al., 2017).
- As adults age, presbycusis (i.e., age-related hearing loss) becomes one of the most common sensory deficits and the third most common chronic health condition. Hearing loss in older adults results from a complicated mix of risk factors: cochlear aging, environmental exposures and lifestyle, genetic and biological predisposition, and/or medical comorbidities (Bowl & Dawson, 2019).
Signs and symptoms associated with hearing loss will vary, and they may include
- avoidance of social settings and/or reduced participation in activities;
- consistent requests for repetition;
- difficulty understanding speech
- in noise,
- of high-pitched speakers, and/or
- on the telephone;
- hearing others’ speech as “mumbled”;
- increasing the volume on the television and other devices;
- listening fatigue;
- perception of “muffled” hearing;
- tinnitus;
- trouble hearing consonants; and
- speaking too loudly or too softly.
Conductive Hearing Loss
Causes of conductive hearing loss include
- absence or malformation of the outer ear, ear canal, or middle ear;
- benign tumors;
- fluid in the middle ear space (e.g., from upper respiratory infection or middle ear infection);
- head trauma (e.g., skull fracture, damage to the tympanic membrane or middle ear structures);
- impacted cerumen;
- infection of the ear canal (e.g., external otitis, swimmer’s ear);
- otitis media (i.e., infection of the middle ear);
- otosclerosis (i.e., reduced vibration of the middle ear bones);
- perforated tympanic membrane;
- poor Eustachian tube function; and
- presence of a foreign body.
Sensorineural Hearing Loss
Causes of sensorineural hearing loss include
- autoimmune inner ear disease (i.e., the immune system mistakenly attacks the inner ear);
- benign tumors (e.g., acoustic neuroma, vestibular schwannoma);
- enlarged vestibular aqueduct syndrome (i.e., a congenital inner ear malformation that can cause vestibular disorders and hearing loss);
- genetic causes, both syndromic (e.g., CHARGE syndrome, Pendred syndrome, Waardenburg syndrome) and nonsyndromic (e.g., genetic mutation);
- head trauma (e.g., inner ear damage, traumatic brain injury);
- infections (e.g., bacterial, viral, parasitic), including
- cytomegalovirus,
- herpes,
- labyrinthitis,
- measles,
- meningitis,
- mumps,
- toxoplasmosis, and
- syphilis;
- Ménière’s disease (i.e., an inner ear disease that can cause vertigo, tinnitus, and fluctuating hearing loss);
- noise exposure;
- ototoxic medications and chemicals (e.g., chemotherapy regimens, aminoglycoside antibiotics);
- presbycusis; and
- vascular deficits (e.g., vertebrobasilar insufficiency, sickle cell anemia; postsurgical).
Mixed Hearing Loss
Mixed hearing loss occurs when there is a combination of one or more causes of conductive hearing loss and one or more causes of sensorineural hearing loss.
Cochlear Synaptopathy/Hidden Hearing Loss
Cochlear synaptopathy, also known as hidden hearing loss, refers to a loss of or damage to nerve connections (i.e., synapses) between the cochlea and the vestibulocochlear nerve/CN VIII. This type of hearing loss is referred to as “hidden” because it is not identified using a standard pure-tone audiometric examination (Barbee et al., 2018). It often results in an individual having trouble understanding speech in noisy environments. Hidden hearing loss may be caused or exacerbated by aging, ototoxic drugs, and noise exposure (Kohrman et al., 2020).
Relationship Between Hearing Loss and Cognition
Approximately one third of Americans aged 65–74 years and nearly half of Americans over the age of 75 years have hearing loss (Villarroel et al., 2019). Many older adults have both hearing loss and cognitive loss, and together, these losses can affect communication, social participation, and quality of life (Pichora-Fuller et al., 2013).
Studies have found hearing loss to be associated with higher rates of cognitive decline and dementia in adults (Lin et al., 2013; Thomson et al., 2017). One hypothesis is that the presence of hearing loss means that greater cognitive resources are dedicated to auditory processing, leaving fewer resources for other cognitive processes, such as working memory (Peelle et al., 2011). Recent research suggests the possibility of a shared etiological pathway responsible for both hearing loss and dementia (Gallacher et al., 2012).
It is important for clinicians to differentiate between hearing loss and cognitive impairment—and to identify when one or both conditions are present. See the ASHA Practice Portal page on Dementia for more information on this topic.
Roles and Responsibilities of Audiologists
Audiologists play a primary role in the screening, assessment, diagnosis, management, and treatment of individuals with hearing loss. Professional roles and activities in audiology include clinical services (diagnosis, assessment, planning, management, and treatment), prevention, advocacy, education, administration, and research. See ASHA’s Scope of Practice in Audiology (ASHA, 2018).
Appropriate roles for audiologists include the following:
- Maintaining knowledge of the anatomy, physiology, and pathophysiology of the auditory and balance systems.
- Remaining informed of research around hearing loss and related disorders.
- Remaining current on new technology (e.g., devices, applications) and accommodations designed to facilitate access for individuals with hearing loss.
- Educating the public and other professionals on hearing loss, the specific needs of individuals with hearing loss, and the role of audiologists in diagnosing and managing hearing loss.
- Serving as an integral member of an interprofessional collaborative team working with individuals with hearing loss and their families.
- Conducting a comprehensive assessment of hearing, auditory function, balance, and related systems.
- Diagnosing the presence of hearing loss and defining the nature of hearing (and related) disorders and their effects on the individual.
- Administering and interpreting tests to differentiate hearing loss from other auditory disorders (e.g., central auditory processing disorder).
- Providing assessment, management, and treatment services for hearing-related disorders (e.g., tinnitus, hyperacusis, misophonia).
- Referring to appropriate professionals to rule out other conditions, to determine etiology, and to facilitate access to comprehensive services.
- Evaluating individuals with hearing loss regarding candidacy for amplification and sensory aids (e.g., hearing aids, cochlear implants) and hearing assistive technology.
- Providing recommendations for the selection, fitting, and dispensing of ear-level amplification (i.e., hearing aids).
- Fitting and maintaining amplification and assistive technology for optimal use.
- Developing and implementing a comprehensive and person-centered audiologic rehabilitative management plan in collaboration with an interprofessional team.
- Counseling individuals with hearing loss and their families on the psychosocial aspects of hearing loss.
- Conducting ongoing evaluation and modification of the audiologic management plan.
- Advocating for the communication needs of all individuals, including advocating for the rights to and funding of services for those with hearing loss and/or related disorders.
- Providing prevention information, promoting hearing wellness, and monitoring acoustic environments.
- Remaining informed of federal and state mandates and initiatives that impact individuals with hearing loss.
- Consulting to industry on the development of products and instrumentation related to the measurement and management of auditory function.
As indicated in the ASHA Code of Ethics (ASHA, 2023), audiologists who serve this population should be specifically educated and appropriately trained to do so.
Roles and Responsibilities of Speech-Language Pathologists
Speech-language pathologists (SLPs) play a role in the identification, screening, assessment, and rehabilitation of individuals with hearing loss. Professional roles and activities in speech-language pathology include clinical services, prevention, advocacy, education, administration, and research. See ASHA’s Scope of Practice in Speech-Language Pathology (ASHA, 2016).
Appropriate roles for SLPs include the following:
- Maintaining general knowledge of the anatomy, physiology, and pathophysiology of the auditory and balance systems and the effects of hearing loss on communication.
- Maintaining general knowledge of amplification devices and hearing assistive technology systems.
- Educating the public and other professionals on the communication needs of individuals with hearing loss.
- Serving as an integral member of an interprofessional collaborative team working with individuals with hearing loss and their families.
- Providing hearing screenings.
- Referring to an audiologist for a comprehensive audiologic evaluation.
- Providing a comprehensive, culturally and linguistically appropriate evaluation of an individual’s speech, language, listening, and communication skills.
- Developing and implementing a comprehensive and person-centered aural rehabilitation plan in collaboration with an interprofessional team.
- Establishing augmentative and alternative communication techniques and strategies, including developing, selecting, and prescribing systems and devices.
- Providing training in the areas of hearing protection and noise hazards; modification of the listening environment; listening and communication behaviors and strategies; and self-advocacy.
- Counseling individuals with hearing loss and their families on the psychosocial aspects of hearing loss.
- Advocating for the communication needs of all individuals, including advocating for the rights to and funding of services for those with hearing loss and/or related disorders.
- Remaining informed of federal and state mandates and initiatives that impact individuals with hearing loss.
As indicated in the ASHA Code of Ethics (ASHA, 2023), SLPs who serve this population should be specifically educated and appropriately trained to do so.
See the Assessment section of the Hearing Loss (Adults) Evidence Map for pertinent scientific evidence, expert opinion, and client/caregiver perspective. For guidance and considerations on infection control practices during the assessment process, see the ASHA page on infection control resources for audiologists and speech-language pathologists.
The purpose of a comprehensive and person-centered audiologic assessment is to
- assess the integrity of the auditory system in each ear,
- measure hearing sensitivities across frequencies,
- determine the type of hearing loss,
- establish a baseline for future monitoring,
- provide ear-specific information needed to initiate amplification device fitting,
- assess the impact of the hearing loss on functionality and quality of life, and
- initiate appropriate individual and family counseling and education.
Audiologic assessment may include
- a case history;
- a recommendation for medical referral, if indicated;
- a cognitive screen;
- otoscopy;
- acoustic immittance procedures, including tympanometry, static immittance, and acoustic reflex measures;
- otoacoustic emissions (OAEs) screening;
- pure-tone audiometry (air conduction, bone conduction);
- speech audiometry in quiet and in noise (e.g., speech thresholds, word recognition measures, speech-in-noise [SIN] tests);
- self-assessments, including communication inventories and needs assessment inventories;
- auditory brainstem response (ABR); and/or
- threshold estimation.
Case History
Accurate diagnosis of hearing loss and/or related disorders relies on the audiologist’s interpretation of a test battery within the context of an individual’s medical and/or developmental history. Case history information may indicate a need for modification of evaluation procedures. For example, the audiologist may evaluate the high-frequency region of the cochlea (above 4000 Hz) for an individual with a history of ototoxic drug exposure or may alter routine assessment procedures for an individual with multiple disabilities.
The specific questions included on a case history may vary on the basis of individual circumstances and may include information related to
- medical history, including
- general health history,
- history of ear infections,
- medication use (prescriptions and/or over-the-counter medications),
- presence of other disabilities,
- previous hearing screening and testing results,
- presence of pain or discharge from ears,
- history of dizziness, balance problems, or falls, and
- presence of tinnitus;
- hearing history and status, including
- when the difficulty with hearing was first noticed,
- whether the onset of hearing difficulty was gradual or sudden,
- whether one ear hears better than the other,
- specific challenges (e.g., frequently asking for repetition, difficulty hearing speech in background noise),
- situational difficulties (e.g., a noisy restaurant, a theater, large groups, while riding in cars), and
- feedback from others (e.g., comments regarding speaking too loudly or turning up the volume on the television);
- family history of hearing loss;
- functional communication skills and/or communication breakdown complaints;
- noise exposure, including during recreational and occupational activities;
- changes in social interaction;
- changes in educational or occupational performance; and
- intervention history (e.g., previous hearing aid trials).
See the ASHA Practice Portal page on Cultural Responsiveness for information on gathering a case history.
Medical Referral
A medical referral may be indicated during, or based upon, a comprehensive audiologic assessment for a variety of reasons, including
- visible traumatic deformity of the ear,
- complaint of pain or discomfort in the ear,
- complaint of acute or chronic dizziness,
- active (or recent history of) drainage from the ear, and/or
- a history of sudden or rapidly progressive hearing loss in the previous 90 days.
Cognitive Screen
Audiologists can incorporate cognitive screening into the comprehensive assessment process (Shen et al., 2016; Souza, 2018). Information on cognitive screening strategies as well as descriptions of various screening tools that may be helpful to audiologists are available (Cordell et al., 2013; Sweetow, 2015).
Audiologists may be in the unique position to uncover an individual’s change or decline in cognitive abilities and, thus, “need to anticipate, identify, and manage mild cognitive impairment in the patients they serve and, perhaps, play a significant role in delaying its onset” (Remensnyder, 2012, p. 25). Referrals to other professionals may be indicated.
Otoscopy
A visual inspection of the pinna and ear canal, including otoscopy, precedes audiometric testing to rule out concerns such as active pathological conditions, the presence of foreign objects, and the potential for ear canal collapse caused by audiometric earphones. The audiologist ensures that the external auditory canal is free of excessive cerumen before testing. A medical referral is made if indicated based on otoscopy findings.
Acoustic Immittance Testing
Acoustic immittance testing is useful in assessing the anatomy of the middle ear and the function of the tympanic membrane and muscle reflexes. If hearing loss is present, this testing helps in localizing the site of lesion. Acoustic immittance testing may include tympanometry and/or acoustic reflex testing.
Tympanometry
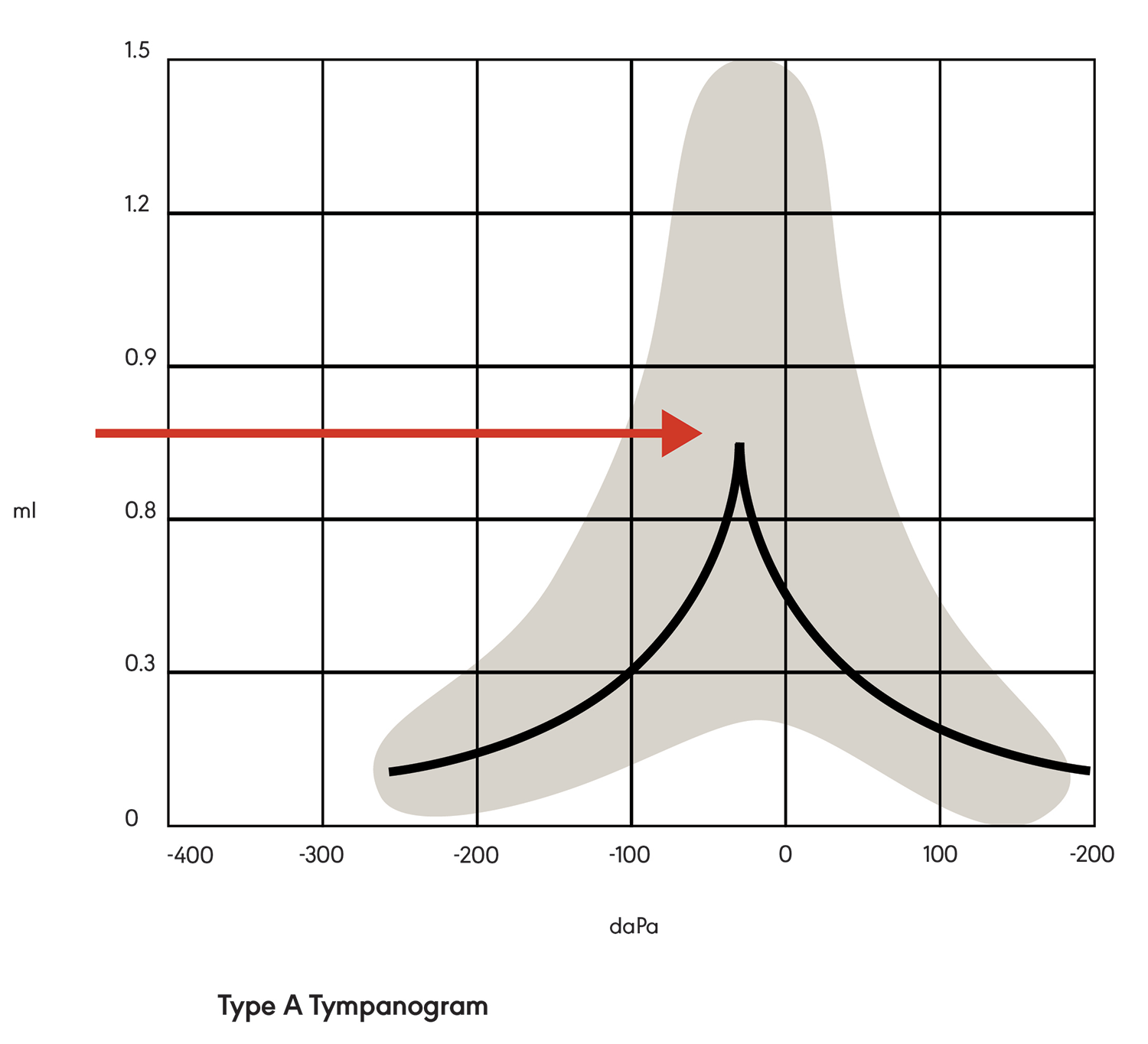
Tympanometry is a measurement of middle ear function. During testing, a tympanometry probe is placed in the ear canal and generates a pure tone. The response (mobility) of the tympanic membrane to this sound is measured at different air pressure levels. The results are represented in a graph called a tympanogram. The middle ear compliance (i.e., response to sound) is plotted vertically on the tympanogram, and air pressure is indicated on the horizontal axis. Maximum compliance of the middle ear system occurs when the pressure in the middle ear cavity is equal to the pressure in the external auditory canal. The maximum compliance value (static acoustic admittance) occurs at the highest peak of the curve on the graph (see Figure 1). Various middle ear pathologies (e.g., otitis media, otosclerosis, tympanic membrane perforation) yield distinctive tympanograms.
There are three main types of tympanograms, identified as A, B, and C. In a Type A tympanogram (see Figure 1), the graph is tent-shaped with peak compliance at or near atmospheric pressure. This indicates a normally functioning middle ear system with nothing (e.g., fluid) preventing the transmission of sound from the middle ear to the cochlea. Type A graphs may have variations (e.g., peak height), indicating abnormalities.
Figure 1. Type A tympanogram showing maximum peak compliance.
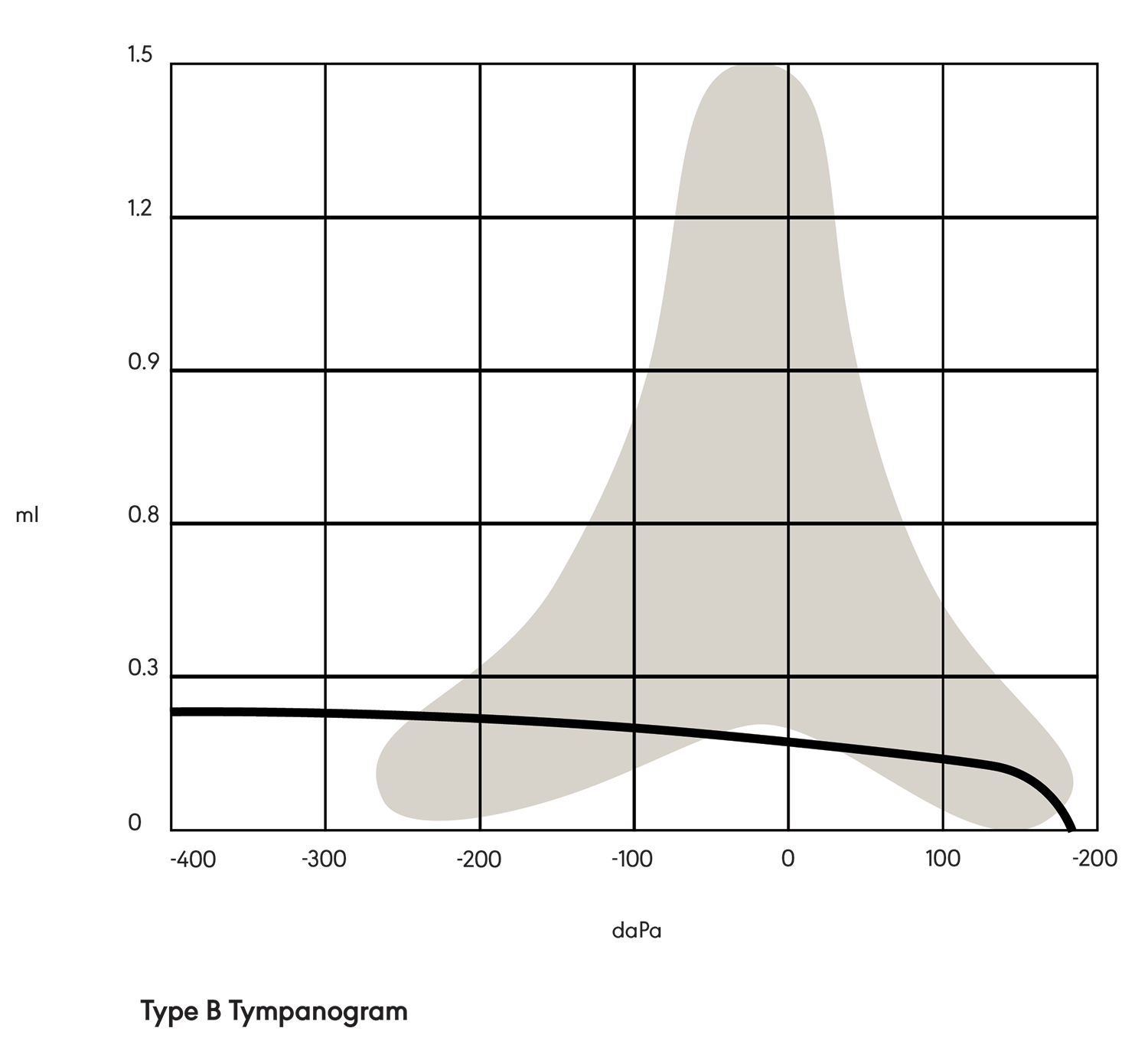
In a Type B tympanogram (see Figure 2), the shape is relatively flat with no sharp peak. This indicates a middle ear pathology, such as fluid behind the tympanic membrane or a perforation in the tympanic membrane.
Figure 2. Type B tympanogram.
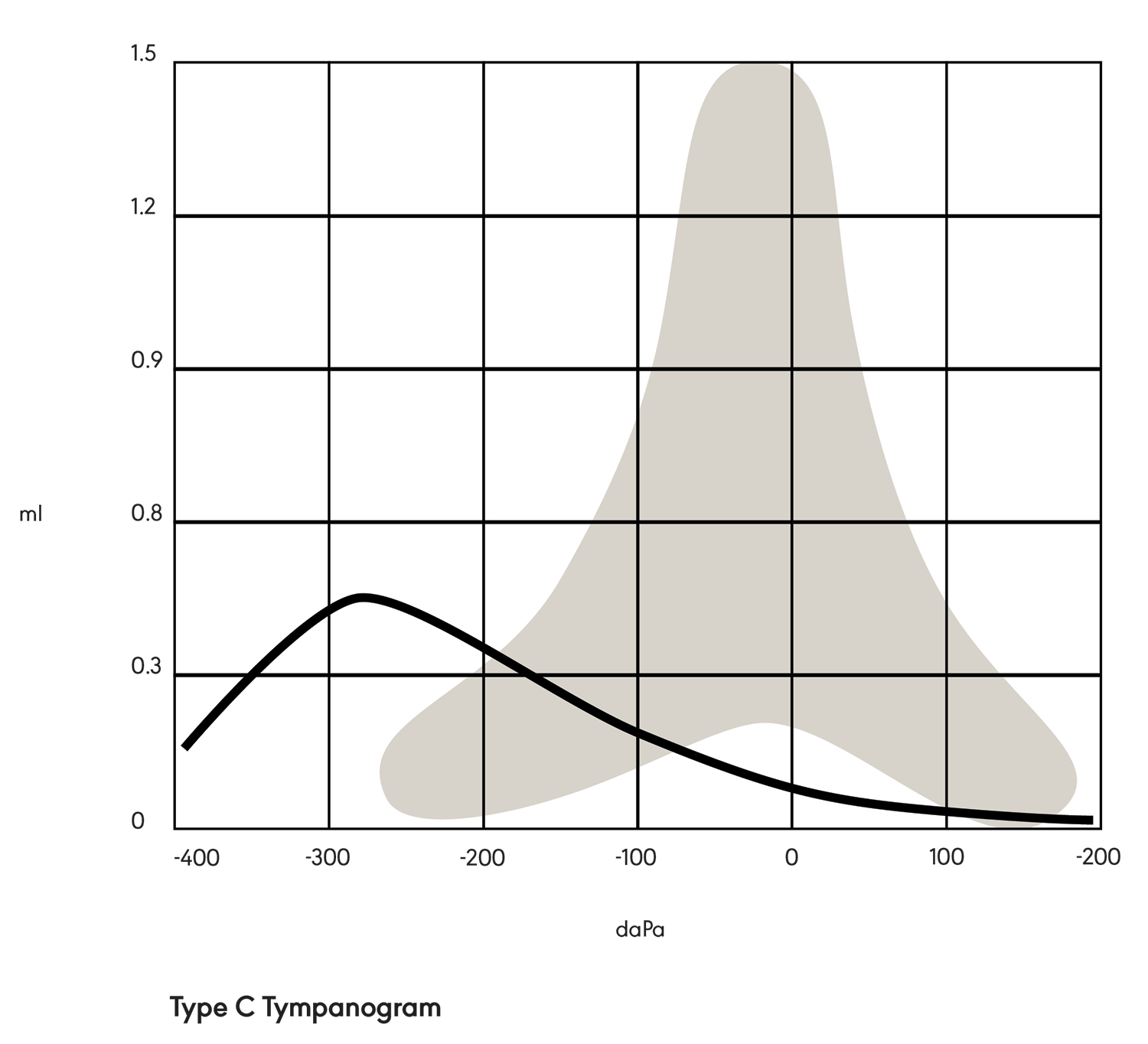
A Type C tympanogram (see Figure 3) is tent-shaped but shifted negatively on the graph. This indicates negative pressure in the middle ear space (e.g., due to allergies, a cold, or otitis media).
Figure 3. Type C tympanogram.
Acoustic Reflexes
The acoustic reflex threshold (ART) is the sound pressure level at which the acoustic reflex will be triggered. Ipsilateral and contralateral evaluation of acoustic reflexes is performed to assess the integrity of the acoustic reflex pathway. ARTs are measured at 500 Hz, 1000 Hz, and 2000 Hz. Testing at 4000 Hz is not recommended because many people with typical hearing have elevated reflexes at this frequency (Gelfand, 1984; Silman & Silverman, 1991).
Normal ARTs range approximately from 85 dB SPL to 100 dB SPL for pure-tone stimuli (Gelfand, 1984). In the case of a conductive pathology, acoustic reflexes will be either absent in the probe ear because the pathology prevents the ability to monitor changes in immittance at the probe tip or elevated because the pure-tone stimulus reaching the cochlea is reduced. In the case of a sensorineural pathology, ARTs are dependent on hearing sensitivity. In the case of a retrocochlear pathology (i.e., involving the vestibulocochlear nerve/CN VIII or other areas of the central auditory system), ARTs are either elevated above what would have been expected in sensorineural hearing loss or absent.
Acoustic reflex decay is the reduction in the magnitude of the acoustic reflex during a prolonged stimulus. It is measured only at 500 Hz and 1000 Hz because rapid adaptation is common at higher stimulus frequencies. Reflex decay is typically measured contralaterally. Reflex decay is abnormal if the reflex is reduced by more than 50% during a 10-s stimulus. Abnormal reflex decay is associated with retrocochlear pathologies.
Otoacoustic Emissions
An OAE is a sound generated from the cochlea in response to an auditory stimulation. The measurement of OAEs is used to assess cochlear function and is helpful in the process of differential diagnosis. Absent OAEs may be a sign of hearing loss or a blockage in the outer or middle ear.
A quiet environment and a snug probe fit are essential for valid and reliable recordings of OAEs. Ears are tested one at a time. Acceptable OAE protocols include the following:
- Transient evoked otoacoustic emissions (TEOAEs) are elicited using one level (e.g., 80 dB pSPL) of click stimuli. Distributions for this condition for normal hearing are documented in the literature (Hussain et al., 1998).
- Distortion product otoacoustic emissions (DPOAEs) are elicited using two pure-tone frequencies simultaneously at either equal or unequal intensities (e.g., 65/55 dB SPL). Distributions for this condition for normal hearing are documented in the literature (Gorga et al., 1997).
Pure-Tone Audiometry
Pure-tone audiometry is a behavioral test of hearing sensitivity. Test results are plotted on a graph called an audiogram—with sound frequency on the horizontal axis and sound intensity on the vertical axis. Data from the right ear and the left ear are plotted separately on the chart. Pure-tone audiometry can be completed using air-conduction or bone-conduction measures.
Air-Conduction Measures
In air-conduction pure-tone audiometry, earphones (supra-aural, circumaural, and insert) are used to present a steady, warble, or pulsed tone signal. Appropriate earphones are dependent upon the situation, are matched to the audiometer, and should not be interchanged without recalibration. Pulsed tones have been shown to increase a test participant’s awareness of the stimuli (Burk & Wiley, 2004). Familiarization with the test tone before threshold measurement is not typically recommended. If the hearing sensitivity in one ear is known to be better, typically, that ear is tested first.
Pure-tone thresholds are measured as the lowest intensity in decibels at which a certain frequency is perceived 50% of the time. Thresholds are typically assessed at frequencies between 250 Hz and 8000 Hz, except when a low-frequency hearing loss exists or is suspected, in which case the hearing threshold at 125 Hz is also measured. The frequency presentation order is not likely to significantly influence test results; however, using a standard order can ensure consistency and minimize the risk of omissions (American National Standards Institute [ANSI], 2018b). When differences of 20 dB or more occur between the threshold values at adjacent octave frequencies, additional frequencies may be tested at the discretion of the clinician (ANSI, 2019). The inclusion of frequencies at 3000 Hz and 6000 Hz in routine testing of air-conduction thresholds may provide the audiologist with a more complete profile of the individual’s hearing status for diagnostic purposes (Fausti et al., 1999; Humes et al., 2006).
Extended high-frequency audiology involves testing frequencies between 9000 Hz and 20000 Hz. This type of testing may be useful in the early detection of hearing loss at high frequencies, especially when there are specific concerns such as exposure to noise or ototoxic chemicals (Hunter et al., 2020; Valiente et al., 2016), and may be clinically relevant as it applies to hearing speech in noise (Motlagh Zadeh et al., 2019).
Air-conduction pure-tone audiometry can be confounded by a crossover or contralateralization of the signal, which occurs when a signal presented to one ear is perceived by the other ear. To address this problem, masking noise can be presented to the non-test ear while the other ear is receiving the stimuli. If masking is used, it is noted on the audiogram.
Bone-Conduction Measures
Bone-conduction audiometry utilizes vibrations of the skull as testing stimuli. Bone oscillators generate vibrations that stimulate the cochlea directly and bypass potential problems in the outer and middle ear. Standard bone conduction vibrator placement is on the mastoid process or forehead with proper force applied (Dirks, 1964). The instruments used must meet the appropriate specifications (ANSI, 2002). The test ear is not covered or occluded for standard bone-conduction measurements. The contralateral ear is covered when masking is being used.
Thresholds are typically assessed at 250 Hz, 500 Hz, 1000 Hz, 2000 Hz, 3000 Hz, and 4000 Hz. Higher frequencies may be tested as indicated and possible. Bone-conducted signals, especially at low frequencies, may elicit vibrotactile responses (Boothroyd & Cawkwell, 1970; Roeser et al., 2007). Suspected vibrotactile responses are noted on the audiogram.
Since the threshold values on which the calibration of bone vibrators is based were measured with masking noise in the contralateral ear, the audiologist may prefer to always use masking in bone-conduction testing. The type and magnitude of the masking used is noted on the audiogram.
Modifications of Pure-Tone Audiometry
Modifications for potential issues encountered during pure-tone audiometry are listed below. The modifications are not intended to be comprehensive in scope or ideal for all situations.
- Claustrophobia in the sound booth may be addressed by leaving the door ajar, seating the individual facing the window, and/or instructing the individual on how to exit the sound booth.
- A collapsed ear canal can be addressed by using insert earphones, supporting the pinna from behind, and/or testing with the individual’s mouth open (Reiter & Silman, 1993).
- The developmental age of the individual may be addressed with age-appropriate test modifications such as visual reinforcement, conditioned play, or computerized audiometry.
- Physical limitations for a motor response can be addressed by using a modified motor task or a verbal response.
- Severe or profound hearing loss can be addressed by starting with low-frequency pure tones.
- Tinnitus can be addressed by using a pulsed signal or a warble tone to help distinguish the test signal from the tinnitus.
Re-instruction, counseling, and re-examination can be used in difficult testing situations. In some cases, alternative objective measures may be used.
Speech Audiometry
Speech audiometry includes speech detection (awareness) thresholds (SDTs), speech recognition thresholds (SRTs), word recognition testing, and SIN testing. Speech audiometry can be used to evaluate hearing sensitivity and speech perception ability as well as for site-of-lesion assessment.
Speech Thresholds
An SDT is the minimum hearing level at which an individual can detect the presence of speech material 50% of the time. The listener does not have to identify the material as speech but must indicate awareness of sound. Common test material for the SDT may be running speech or familiar words.
An SRT is the minimum hearing level (ANSI, 2018b) at which an individual can recognize (e.g., correctly repeat) 50% of speech material. SRTs are measured separately in each ear. Spondees (two-syllable words with equal stress on both syllables) are the typical test material used to determine the SRT.
Examples of techniques for establishing an SRT (as described by Stach & Ramachandran, 2021) have been delineated by Downs and Minard (1996) and Huff and Nerbonnet (1982). Both methods start by familiarizing an individual with the given spondees. The methods differ in the initial presentation level (e.g., 30 dB below a previously established SRT or 30 dB above the estimated threshold); the approach used for threshold seeking (e.g., ascend in 10-dB steps until a correct response and then descend 15 dB or increase by 20 dB if the initial response is incorrect and then decrease the level by 10 dB); and the number of correct responses required to obtain the SRT.
General Considerations for Clinical Determination of the Speech Threshold
- Documentation of the specific type of test material (i.e., stimuli) used helps ensure test–retest reliability and may be useful information for future hearing evaluations.
- When circumstances or individual capabilities prevent the determination of the SRT, the SDT may be determined instead. The SDT will occur at a lower level than the SRT because the SDT depends on audibility alone, whereas the SRT requires that an individual both hear and identify the speech signal. The threshold of detection can be expected to be approximately 5–10 dB better than the threshold of recognition.
- For assessing the SDT, several response modes can be used to convey signal detection. Usually, these response modes are nonverbal.
- The usual response mode for obtaining the SRT is repetition of the stimulus item. In cases where it is not possible to obtain verbal responses, alternative response modes are needed. Acceptable alternatives must convey the recognition of test items from a closed set of choices (e.g., picture pointing, signing, visual scanning). If a picture-pointing task is used, then the clinician should be cautious in choosing the number of response items. Too few items increase the probability of chance performance, and too many items may be distracting and may increase response time (e.g., a number between eight and 12 words is usually appropriate).
- The basic procedure for determining SRTs consists of instructions, familiarization, a single series of descending threshold determinations, and calculation of threshold hearing level.
- Either a recorded or a monitored live voice technique can be used to obtain the speech threshold. Recorded presentation of the test material is the preferred procedure. When monitored live voice is used, it should be noted with the test results.
- Masking of the non-test ear may be used as appropriate when measuring an SDT or SRT. The appropriate masker for a speech stimulus must have a wideband spectrum (e.g., white noise or speech-spectrum noise). The level of effective masking used should be sufficient to eliminate reception by the non-test ear without causing overmasking.
- In most cases, there is a high correlation between speech thresholds and the pure-tone average. Speech thresholds can serve as a cross-check of a pure-tone audiogram. Disagreement between the two indicates inconsistent test results, which may result from pseudohypacusis (i.e., exaggerated or false hearing loss) or various testing variables (e.g., misunderstanding instructions, equipment malfunction).
- Behavioral, cognitive, and language issues may impact testing results.
Word Recognition
Word recognition scores are obtained for each ear using phonetically balanced monosyllabic words and are expressed as a percent correct. A variety of test word lists are available.
Testing may be terminated after 10 words with a modified word list arranged by difficulty, if no errors occur, or after 25 words, if there are no more than four errors. Otherwise, the full 50-item list is administered (Runge & Hosford-Dunn, 1985).
Either a recorded or a monitored live voice technique can be used to obtain the word recognition score. Recorded presentation of the test material is the preferred procedure, as it standardizes the composition and presentation of the test list, allows for better control of the intensity of the test items, and ensures that the speech pattern of the recorded talker is consistent. When monitored live voice is used, it is noted in the test results.
Masking is used for word recognition testing when the presentation level in the test ear exceeds the best bone conduction threshold in any of the speech frequencies (i.e., 500 Hz, 1000 Hz, or 2000 Hz) for the non-test ear by 35 dB or more (Roeser et al., 2007).
Specific considerations are given when evaluating the speech recognition skills of individuals who are non-English speakers and English learners. It is preferable to secure the services of a bilingual audiologist to appropriately administer and monitor tests developed in other languages, whether presented in live voice or prerecorded. The services of a trained interpreter/translator are indicated when working with monolingual audiologists to obtain the most accurate word recognition scores. It is not appropriate for either the bilingual audiologist or the trained interpreter/translator to simply translate English materials. Test materials must reflect the phonetic balance of the individual’s language. Reducing the test set size by including only familiar words may result in inaccurate threshold measurements. Alternative methods of measuring speech recognition have been developed, such as using paired digits as stimuli. See the ASHA Practice Portal page on Collaborating With Interpreters, Transliterators, and Translators for more information.
Sin Testing
For many people with hearing loss, a main complaint is difficulty understanding speech in background noise. The results of speech testing in background noise may be quite different from results obtained in a quiet environment. SIN testing can provide information about an individual’s hearing in conditions representative of real-world situations.
When completing SIN testing, the audiologist must consider stimulus (e.g., words, sentences), presentation level(s), noise type(s), and signal-to-noise ratio (SNR). The clinician may keep the SNR fixed during testing, with the speech intensity level and noise intensity level remaining the same throughout. However, in an adaptive approach to SIN testing, the clinician systematically changes the intensity of the speech or the noise during testing and pinpoints the SNR where communication begins to be impacted. A variety of standardized test materials are available for SIN testing.
Self-Assessments
Several self-assessments are available to measure the impact of hearing loss on an individual’s everyday life and to provide individualized assessment of situations that cause communication difficulty. Measuring the extent to which a hearing loss is limiting or restrictive provides the audiologist with additional information about the individual’s quality of life, communication needs, and personal motivation. These measurements can also serve as outcome measures or as baseline assessment against which to compare the eventual benefits of management and/or treatment (e.g., hearing aids). See the ASHA resource on self-test for hearing loss.
ABR Testing
ABR testing can be used to detect hearing loss and estimate auditory thresholds in cases where the completion of other testing (e.g., behavioral) is difficult. ABR testing can also be used for a differential diagnosis of cochlear hearing loss versus retrocochlear hearing loss (e.g., caused by tumors of the vestibulocochlear nerve/CN VIII). The procedure involves placing electrodes on the skin in specified areas and then administering sound stimuli (e.g., clicks) through insert earphones. Auditory evoked potentials (i.e., evoked responses to auditory stimuli) originating from the vestibulocochlear nerve/CN VIII and auditory brainstem structures can be recorded on a waveform that typically consists of five to seven identifiable peaks. The peaks represent neural function of the auditory pathways and nuclei. Both latency measures and amplitude measures can be derived from the ABR waveform. Abnormalities in these measurements may be associated with pathology.
Threshold Estimation
When behavioral audiometric tests are judged to be unreliable, when ear-specific thresholds cannot be obtained, or when results are inconclusive regarding type, degree, or configuration of hearing levels, the audiologist may use objective threshold estimation, such as ABR or auditory steady-state response (ASSR), as part of the assessment process to predict hearing thresholds.
Frequency-Specific ABR
Predicting hearing sensitivity using ABR involves determining the lowest intensity level at which an auditory evoked potential can be identified. Click or tone-burst stimuli (at low, mid, and high frequencies) are presented at an intensity level that evokes a response. The intensity level is then progressively lowered, and the responses tracked, until an intensity is reached at which the response is no longer observable. This intensity level corresponds closely to a behavioral hearing threshold.
ASSR
The auditory steady-state response (ASSR) is an auditory evoked potential, elicited with amplitude- and frequency-modulated tones, that can be used to predict hearing sensitivity in individuals of all ages (Dimitrijevic et al., 2002; Rance & Rickards, 2002). The response is an evoked neurological potential in response to the periodic modulation of a tone. It can be detected objectively at intensity levels close to behavioral thresholds. The ASSR can yield a clinically acceptable, frequency-specific prediction of behavioral thresholds.
Test Environment and Equipment Calibration
The accuracy of the audiologic assessment process is dependent upon maintaining appropriate specifications regarding the testing environment and equipment calibration. It is essential that all audiometric equipment be calibrated, be functioning properly, and be used in an acceptable test environment to ensure accurate test results (ANSI, 2012, 2018a, 2018b).
Exhaustive electroacoustic calibrations should be performed on a regular basis (e.g., annually) using instrumentation traceable to the National Institute of Standards and Technology. Functional inspection, performance checks, and bioacoustic checks may be conducted daily to verify equipment performance prior to use.
See the Treatment section of the Hearing Loss (Adults) Evidence Map for pertinent scientific evidence, expert opinion, and client/caregiver perspective. For guidance and considerations on infection control practices during the treatment process, see the ASHA page on infection control resources for audiologists and speech-language pathologists.
Treatment Planning
At the completion of the assessment process, the audiologist, the individual seeking services, and their family review the findings and identify areas of need. Priorities and specific goals for intervention are jointly agreed upon, with the individual who is seeking services being at the center of the decision-making process. Treatment planning may include counseling and recommendations for amplification, aural rehabilitation (AR), hearing assistive technology systems (HATS), and/or other professional services as appropriate.
International Classification of Functioning, Disability and Health
The World Health Organization (WHO, 2001) published the International Classification of Functioning, Disability and Health as a classification of health and health-related domains with consideration of disability, functional status, and environmental factors. This classification system can be used to assist clinicians in patient care management, both in establishing goals and in determining specific outcomes that can be measured through patient report. See the ASHA resource on the International Classification of Functioning, Disability, and Health (ICF) for more information.
Management and Treatment Options
A comprehensive and person-centered management and/or treatment plan for an adult with hearing loss may include, but not be limited to, the following interventions.
Medical Treatment
In some cases, hearing loss is caused by a medical condition or an event requiring medical or surgical management (e.g., tumor, trauma, infection). Audiologists refer to the appropriate medical and/or related professionals as indicated.
Aural Rehabilitation
Boothroyd (2007) defined aural rehabilitation (AR) as “the reduction of hearing-loss-induced deficits of function, activity, participation, and quality of life through a combination of sensory management, instruction, perceptual training, and counseling” (p. 63). A comprehensive, person- and family-centered AR plan, possibly involving a collaborative interprofessional team of professionals, may include
- amplification devices and related education and training,
- HATS,
- informational and personal adjustment counseling,
- recommendations for modifications to room acoustics or lighting,
- referrals to speech-language pathologists for speech and/or voice production concerns, and
- training in a variety of areas and selected modalities to maximize communication skills in environments relevant to the individual receiving services (e.g., auditory training, communication skills training).
See the ASHA Practice Portal page on Aural Rehabilitation for Adults for detailed information.
Amplification
A comprehensive plan of care for an individual with hearing loss may include the selection and fitting of a sensory device or the maximization of a current device (e.g., hearing aid, bone conduction device, cochlear implant, implantable device). This includes instruction and education on the effective use and appropriate care of the device as well as counseling on the adjustment to the device and realistic expectation for benefit.
See the ASHA Practice Portal pages on Cochlear Implants and Hearing Aids For Adults for detailed information.
HATS
Hearing assistive technologies systems (HATS) include a variety of devices designed to improve audibility in specific listening situations. HATS may be designed to be used independently or in conjunction with hearing aids or cochlear implants. They may be intended for personal or group use.
Examples of hearing assistive technology include the following:
- An alerting device provides a signal (e.g., strobe light, regular light, vibration) to alert an individual with a hearing loss that a sound has occurred (e.g., doorbell, telephone, smoke alarm, clock alarm, watch alarm, baby monitor).
- A frequency modulation system uses radio waves to transmit sound from the source to a receiver worn by an individual.
- An induction loop system uses hard-wire loops placed under floors or around walls to convert sound to magnetic forces, which cochlear implants and hearings aids with telephone switches or t-coils can convert back to sound.
- An infrared system uses infrared waves to send sound to the listener’s infrared receiver.
- A personal amplification system amplifies sound with systems such as streamers (Bluetooth), mini remote microphones, and digital applications.
- A telephone amplifier amplifies speech heard over the phone.
- A television assistive device and/or accessories use wireless streaming or a loop system to make TV volume more comfortable and clearer.
- A text telephone (TTD or TTY) sends and receives typed messages through telephone lines.
- A voice carryover telephone connects an individual with hearing loss to a local relay service.
Counseling and Education
Counseling and education for individuals with hearing loss and their family begins during the initial visit and continues throughout the entire diagnostic, treatment, and management process. Counseling an individual with hearing loss requires providing information that is clear, understandable, and in a health literate format. See the ASHA resource on health literacy for more information.
A number of handouts that may be helpful during patient and family counseling activities are available at the ASHA page for audiology patient education handouts. For more information, see the ASHA Practice Portal page on Counseling For Professional Service Delivery.
Potential topics for counseling and education include
- the nature of the hearing loss and its effects on communication and quality of life;
- specific interpersonal, psychosocial, educational, and vocational implications of hearing loss for the individual and their family;
- the use of effective coping and compensatory skills to minimize the effect of the hearing loss; and
- self-advocacy and self-management of hearing loss.
Service Delivery
See the Service Delivery section of the Hearing Loss (Adults) Evidence Map for pertinent scientific evidence, expert opinion, and client/caregiver perspective.
Service delivery variables that may have an impact on treatment outcomes include dosage, format, provider, timing, and setting.
Reimbursement
Regardless of the payment source(s), patients must be offered the same services, the cost of the services must be equitable, and national procedure codes must be used for requesting reimbursement. For more information, see the ASHA resource on billing and reimbursement.
ASHA Resources
- ASHA State-by-State
- Autoimmune Inner Ear Disease
- Billing and Reimbursement
- Health Literacy
- Hearing-Related Topics: Terminology Guidance
- Infection Control Resources for Audiologists and Speech-Language Pathologists
- International Classification of Functioning, Disability, and Health (ICF)
- Interprofessional Education/Interprofessional Practice (IPE/IPP)
- Ototoxic Medications (Medication Effects)
- Patient Education Handouts—Audiology Information Series
- Person-Centered Care in Audiology
- Self-Test for Hearing Loss
Other Resources
This list of resources is not exhaustive, and the inclusion of any specific resource does not imply endorsement from ASHA.
- Association of Late-Deafened Adults
- Hearing Loss Association of America
- Ida Institute
- International Classification of Functioning, Disability and Health (ICF)
- Institute for Patient- and Family-Centered Care
- National Institute on Deafness and Other Communication Disorders (NIDCD)
- National Institute for Occupational Safety and Health (NIOSH): Criteria for a Recommended Standard on Occupational Noise Exposure [PDF]
- U.S. Department of Labor Occupational Safety and Health Administration (OSHA): Standards on Occupational Noise Exposure
- World Health Organization - International Telecommunication Union: Standard on Safe Listening Devices and Systems [PDF]
American National Standards Institute. (2002). Mechanical coupler for measurement of bone vibrators (Rev. ed.) (ANSI S3.13-1987). Acoustical Society of America.
American National Standards Institute. (2012). Specifications for instruments to measure aural acoustic impedance and admittance (aural acoustic immittance) (Rev. ed.) (ANSI S3.39-1987). Acoustical Society of America.
American National Standards Institute. (2018a). Maximum permissible ambient noise levels for audiometric test rooms (Rev. ed.) (ANSI S3.1-1999). Acoustical Society of America.
American National Standards Institute. (2018b). Specification for audiometers (Rev. ed.) (ANSI/ASA S3.6-2018). Acoustical Society of America.
American National Standards Institute. (2019). Methods for manual pure-tone threshold audiometry (Rev. ed.) (ANSI S3.21-2004). Acoustical Society of America.
American Speech-Language-Hearing Association. (2016). Scope of practice in speech-language pathology [Scope of practice]. https://www.asha.org/policy/
American Speech-Language-Hearing Association. (2018). Scope of practice in audiology [Scope of practice]. https://www.asha.org/policy/
American Speech-Language-Hearing Association. (2023). Code of ethics [Ethics]. https://www.asha.org/policy/
Barbee, C. M., James, J. A., Park, J. H., Smith, E. M., Johnson, C. E., Clifton, S., & Danhauer, J. L. (2018). Effectiveness of auditory measures for detecting hidden hearing loss and/or cochlear synaptopathy: A systematic review. Seminars in Hearing, 39(2), 172–209. https://doi.org/10.1055/s-0038-1641743
Boothroyd, A. (2007). Adult aural rehabilitation: What is it and does it work? Trends in Amplification, 11(2), 63–71. https://doi.org/10.1177/1084713807301073
Boothroyd, A., & Cawkwell, S. (1970). Vibrotactile thresholds in pure tone audiometry. Acta Oto-Laryngologica, 69(1–6), 381–387. https://doi.org/10.3109/00016487009123382
Bowl, M. R., & Dawson, S. J. (2019). Age-related hearing loss. Cold Spring Harbor Perspectives in Medicine, 9(8), a033217.
Burk, M. H., & Wiley, T. L. (2004). Continuous versus pulsed tones in audiometry. American Journal of Audiology, 13(1), 54–61. https://doi.org/10.1044/1059-0889(2004/008)
Carroll, Y. I., Eichwald, J., Scinicariello, F., Hoffman, H. J., Deitchman, S., Radke, M. S., Themann, C. L., & Breysse, P. (2017). Vital signs: Noise-induced hearing loss among adults—United States 2011–2012. Morbidity and Mortality Weekly Report, 66(5), 139. https://doi.org/10.15585/mmwr.mm6605e3
Chen, K. H., Su, S. B., & Chen, K. T. (2020). An overview of occupational noise-induced hearing loss among workers: Epidemiology, pathogenesis, and preventive measures. Environmental Health and Preventive Medicine, 25(1), 1–10. https://doi.org/10.1186/s12199-020-00906-0
Clark, J. G. (1981). Uses and abuses of hearing loss classification. Asha, 23(7), 493–500.
Cordell, C. B., Borson, S., Boustani, M., Chodosh, J., Reuben, D., Verghese, J., Thies, W., Fried, L. B., & Medicare Detection of Cognitive Impairment Workgroup. (2013). Alzheimer’s Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting. Alzheimer’s & Dementia, 9(2), 141–150. https://doi.org/10.1016/j.jalz.2012.09.011
Dimitrijevic, A., John, M. S., Van Roon, P., Purcell, D. W., Adamonis, J., Ostroff, J., Nedzelski, J. M., & Picton, T. W. (2002). Estimating the audiogram using multiple auditory steady-state responses. Journal of the American Academy of Audiology, 13(4), 205–224. https://doi.org/10.1055/s-0040-1715964
Dirks, D. (1964). Factors related to bone conduction reliability. Archives of Otolaryngology, 79(6), 551–558. https://doi.org/10.1001/archotol.79.6.551
Downs, D., & Minard, P. D. (1996). A fast valid method to measure speech-recognition threshold. Hearing Journal, 49, 39–44.
Fausti, S. A., Henry, J. A., Helt, W. J., Phillips, D. S., Frey, R. H., Noffsinger, D., Larson, V. D., & Fowler, C. G. (1999). An individualized, sensitive frequency range for early detection of ototoxicity. Ear and Hearing, 20(6), 497–505.
Gallacher, J., Ilubaera, V., Ben-Schlomo, Y., Bayer, A., Fish, M., Babisch, W., & Elwood, P. (2012). Auditory threshold, phonologic demand, and incident dementia. Neurology, 79(15), 1583–1590. https://doi.org/10.1212/WNL.0b013e31826e263d
Gelfand, S. A. (1984). The contralateral acoustic-reflex threshold. In S. Silman (Ed.), The acoustic reflex: Basic principles and clinical applications (pp. 138–186). Academic Press.
Gorga, M. P., Neely, S. T., Ohlrich, B., Hoover, B., Redner, J., & Peters, J. (1997). From laboratory to clinic: A large scale study of distortion product otoacoustic emissions in ears with normal hearing and ears with hearing loss. Ear and Hearing, 18(6), 440–455.
Grenness, C., Hickson, L., Laplante-Lévesque, A., & Davidson, B. (2014). Patient-centred care: A review for rehabilitative audiologists. International Journal of Audiology, 53(Suppl. 1), S60–S67. https://doi.org/10.3109/14992027.2013.847286
Huff, S. J., & Nerbonnet, M. A. (1982). Comparison of the American Speech-Language-Hearing Association and revised Tillman-Olsen methods for speech threshold measurement. Ear and Hearing, 3(6), 335–339.
Humes, L. E., Joellenbeck, L. M., & Durch, J. S. (Eds.). (2006). Noise and military service: Implications for hearing loss and tinnitus. The National Academies Press.
Hunter, L. L., Monson, B. B., Moore, D. R., Dhar, S., Wright, B. A., Munro, K. J., Motlagh Zadeh, L., Blankenship, C. M., Stiepan, S. M., & Siegel, J. H. (2020). Extended high frequency hearing and speech perception implications in adults and children. Hearing Research, 397, 107922. https://doi.org/10.1016/j.heares.2020.107922
Hussain, D. M., Gorga, M. P., Neely, S. T., Keefe, D. H., & Peters, J. (1998). Transient evoked otoacoustic emissions in patients with normal hearing and in patients with hearing loss. Ear and Hearing, 19(6), 434–449.
Kohrman, D. C., Wan, G., Cassinotti, L., & Corfas, G. (2020). Hidden hearing loss: A disorder with multiple etiologies and mechanisms. Cold Spring Harbor Perspectives in Medicine, 10(1), a035493.
Lie, A., Engdahl, B., Hoffman, H. J., Li, C. M., & Tambs, K. (2017). Occupational noise exposure, hearing loss, and notched audiograms in the HUNT Nord-Trøndelag hearing loss study, 1996–1998. The Laryngoscope, 127(6), 1442–1450. https://doi.org/10.1002/lary.26256
Lin, F. R., Yaffe, K., Xia, J., Xue, Q., Harris, T. B., Purchase-Helzner, E., Satterfield, S., Ayonayon, H. N., Ferrucci, L., & Simonsick, E. M. (2013). Hearing loss and cognitive decline among older adults. JAMA Internal Medicine, 173(4), 293–299.
Motlagh Zadeh, L., Silbert, N. H., Sternasty, K., Swanepoel, D. W., Hunter, L. L., & Moore, D. R. (2019). Extended high-frequency hearing enhances speech perception in noise. Proceedings of the National Academy of Sciences, 116(47), 23753–23759. https://doi.org/10.1073/pnas.1903315116
National Center for Environmental Health. (2018, December 11). Statistics about the public health burden of noise-induced hearing loss. Centers for Disease Control and Prevention. https://www.cdc.gov/nceh/hearing_loss/public_health_scientific_info.html
National Center for Health Statistics. (2018, August 27). Crude percentages of hearing trouble for adults aged 18 and over, United States, 2015–2018. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/nhis/ADULTS/www/index.htm
Peelle, J. E., Troiani, V., Grossman, M., & Wingfield, A. (2011). Hearing loss in older adults affects neural systems supporting speech comprehension. The Journal of Neuroscience, 31(35), 12638–12643. https://doi.org/10.1523/JNEUROSCI.2559-11.2011
Pichora-Fuller, M. K., Dupuis, K., Reed, M., & Lemke, U. (2013). Helping older people with cognitive decline communicate: Hearing aids as part of a broader rehabilitation approach. Seminars in Hearing, 34(4), 308–330. https://doi.org/10.1055/s-0033-1356643
Rance, G., & Rickards, F. (2002). Prediction of hearing threshold in infants using auditory steady-state evoked potentials. Journal of the American Academy of Audiology, 13(5), 236–245. https://doi.org/10.1055/s-0040-1715967
Reiter, L. A., & Silman, S. (1993). Detecting and remediating external meatal collapse during audiologic assessment. Journal of the American Academy of Audiology, 4(4), 264–268.
Remensnyder, L. (2012). Audiologists as gatekeepers and it’s not just for hearing loss. Audiology Today, 24(4), 24–31.
Roeser, R. J., Valente, M., & Hosford-Dunn, H. (2007). Audiology: Diagnosis (2nd ed.). Thieme.
Runge, C. A., & Hosford-Dunn, H. (1985). Word recognition performance with modified CID W-22 word lists. Journal of Speech, Language, and Hearing Research, 28(3), 355–362. https://doi.org/10.1044/jshr.2803.355
Scarinci, N., Meyer, C., Ekberg, K., & Hickson, L. (2013). Using a family-centered care approach in audiologic rehabilitation for adults with hearing impairment. Perspectives on Aural Rehabilitation and Its Instrumentation, 20(3), 83–90. https://doi.org/10.1044/arri20.3.83
Sharma, R. K., Lalwani, A. K., & Golub, J. S. (2020). Prevalence and severity of hearing loss in the older old population. JAMA Otolaryngology—Head & Neck Surgery, 146(8), 762–763. https://doi.org/10.1001/jamaoto.2020.0900
Sheffield, A. M., & Smith, R. J. (2019). The epidemiology of deafness. Cold Spring Harbor Perspectives in Medicine, 9(9), a033258.
Shen, J., Anderson, M. C., Arehart, K. H., & Souza, P. E. (2016). Using cognitive screening tests in audiology. American Journal of Audiology, 25(4), 319–331. https://doi.org/10.1044/2016_AJA-16-0032
Silman, S., & Silverman, C. A. (1991). Auditory diagnosis: Principles and applications. Academic Press.
Souza, P. E. (2018). Cognition and hearing aids: What should clinicians know? Perspectives of the ASHA Special Interest Groups, 3(6), 43–50. https://doi.org/10.1044/persp3.SIG6.43
Stach, B. A., & Ramachandran, V. (2021). Clinical audiology: An introduction (3rd ed.). Plural Publishing.
Sweetow, R. (2015). Screening for cognitive disorders in older adults in the audiology clinic. Audiology Today, 27(4), 38–43.
Thomson, R. S., Auduong, P., Miller, A. T., & Gurgel, R. K. (2017). Hearing loss as a risk factor for dementia: A systematic review. Laryngoscope Investigative Otolaryngology, 2(2), 69–79. https://doi.org/10.1002/lio2.65
Valiente, A. R., Fidalgo, A. R., Villarreal, I. M., & Berrocal, J. R. G. (2016). Extended high-frequency audiometry (9000–20000 Hz). Usefulness in audiological diagnosis. Acta Otorrinolaringologica (English Edition),67(1), 40–44. https://doi.org/10.1016/j.otoeng.2015.02.001
Villarroel, M. A. B. D., Blackwell, D. L., & Jen, A. (2019). Tables of summary health statistics for U.S. adults: 2018 National Health Interview Survey. National Center for Health Statistics. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2018_SHS_Table_A-6.pdf [PDF]
World Health Organization. (2001). International Classification of Functioning, Disability and Health.
World Health Organization. (2018). WHO global estimates on prevalence of hearing loss [PowerPoint slides]. https://www.who.int/deafness/Global-estimates-on-prevalence-of-hearing-loss-Jul2018.pptx?ua=1
World Health Organization. (2021a, March 2). WHO: 1 in 4 people projected to have hearing problems by 2050. [News release]. https://www.who.int/news/item/02-03-2021-who-1-in-4-people-projected-to-have-hearing-problems-by-2050
World Health Organization. (2021b, April 1). Deafness and hearing loss [Fact sheet]. https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss
Acknowledgments
Content for ASHA’s Practice Portal is developed through a comprehensive process that includes multiple rounds of subject matter expert input and review. ASHA extends its gratitude to the following subject matter experts who were involved in the development of the Hearing Loss in Adults page.
- Carmen Brewer, PhD, CCC-A
- Karen Farmer, MS
- Susan Gordon-Hickey, PhD, CCC-A
- Brian Kreisman, PhD, CCC-A
- Gail Linn, AuD, CCC-A
- Margaret McCabe, AuD, CCC-A
- Robert Moore, AuD, CCC-A
- Meaghan Reed, AuD, CCC-A
- Carrie Spangler, AuD, CCC-A
In addition, ASHA thanks the members of the Working Group on Manual Pure-Tone Threshold Audiometry whose work on the Guidelines was foundational to the development of this content. Members of the working group were John Campbell, Jeffrey Graley, Deanna Meinke, Linda Vaughan (ex officio), and Ted Madison (chair). Roberta Aungst, ASHA vice president for professional practices in audiology (2004–2007), served as monitoring vice president.
In addition, ASHA thanks the members of the Committee on Audiologic Evaluation whose work on the Guidelines for Determining Threshold Level for Speech was foundational to the development of this content. Members of the committee were Martin S. Robinette (past chair), Carmen C. Brewer, Margaret F. Carlin, John D. Durrant, Thomas A. Frank, Gregg D. Givens, Michael P. Gorga, Carol Kamara (ex officio), Robert H. Margolis, Laura Ann Wilber, and Gilbert H. Herer (vice president for clinical affairs).
Citing Practice Portal Pages
The recommended citation for this Practice Portal page is:
American Speech-Language-Hearing Association (n.d.). Hearing Loss in Adults (Practice Portal). Retrieved month, day, year, from /Practice-Portal/Clinical-Topics/Hearing-Loss/.
Content Disclaimer: The Practice Portal, ASHA policy documents, and guidelines contain information for use in all settings; however, members must consider all applicable local, state and federal requirements when applying the information in their specific work setting.












