Understanding Usher Syndrome
December 2009
Josara Wallber, AuD, CCC-A
Usher syndrome is a genetic condition involving sensorineural hearing loss and retinitis pigmentosa (RP). Although considered a rare disease, it is the most frequent cause of deaf-blindness in humans. Newborn hearing screening has reduced the age of identification of children with hearing loss from 12–18 months to 6 months or less (Harrison & Rousch, 1996), but the diagnosis of Usher syndrome, with its devastating vision loss, typically lags 5–10 years behind the identification of the hearing loss (Kimberling & Lindenmuth, 2007). So, while parents learn of their child's hearing loss relatively early, without a differential diagnosis they proceed with critical decisions related to intervention, communication, and educational options unaware that their child will eventually be blind. Because of newborn hearing screening, audiologists are often the family's primary contact. Therefore, audiologists are in a position to improve differential diagnosis outcomes for children with Usher syndrome. This article is aimed at increasing audiologists' understanding of the audiologic and visual presentation, diagnostic criteria, and intervention strategies involved with Usher syndrome.
Clinical and Genetic Features
Usher syndrome is named after Charles Usher, a British ophthalmologist who described the nature of the disease in 1914. Because it is autosomal recessive, all forms of Usher syndrome are inherited from not one but both parents. As can be seen in Figure 1, when both parents are asymptomatic carriers of the Usher syndrome gene, they have a 25% chance with each pregnancy of producing a child with Usher syndrome. For this reason, you will rarely see families with a history of Usher syndrome. The parents are surprised by the diagnosis and frequently respond that it cannot be genetic because it is not in their family. Estimates are that 1 in 10 persons carries some form of recessive gene for Usher syndrome (W. Kimberling, personal communication, 2009), but because there are many different genetic versions of Usher syndrome there is only a small chance of a specific carrier mating with an identical carrier. Obviously, carriers of two differing Usher genes will produce additional carriers but no affected offspring.
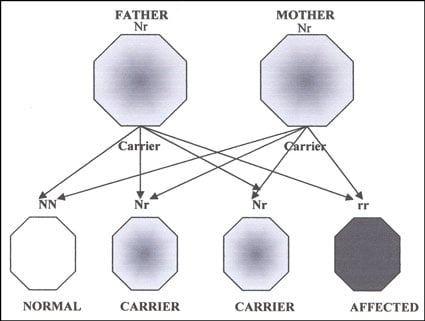
Figure 1. Autosomal recessive inheritance pattern where both parents are unaffected carriers (N = normal, r = recessive trait).
When hearing loss is accompanied by other clinical findings, it is often classified as a syndrome, and there are more than 400 such syndromes outlined by Gorlin, Torell, and Cohen (1995). Our understanding of, and ability to test for, the underlying etiology of syndromes has been greatly enhanced by the study of the human genome and proteins necessary for normal development. Genetic testing is available for many disorders and presents ethical and economic challenges for today's practitioner. While many syndromes may be identified clinically, this strategy has resulted in a significant delay in the diagnosis of Usher syndrome. For a brief overview of the more common syndromic hearing losses, see Keats (2002).
Although it is highly heterogenic, there are least 10 genes that cause Usher syndrome (W. Kimberling, personal communication, 2009), and there are three phenotypic or clinical presentation types generally recognized. As can be seen in Table 1, the types are further divided into distinct molecular subgroups. The distinction between the types is made primarily by severity of hearing loss, presence or absence of vestibular function, and onset of vision loss. It is important to consider all these variables, as hearing loss alone cannot indicate Usher syndrome nor differentiate clearly between the subtypes (Wagenaar, Snik, Kimberling, & Cremers, 1996).
Table 1. Traditional clinical classification of Usher syndrome, including subtypes, genes, hearing loss, vestibular status, and typical onset of night blindness (Cohen, Bitner-Glindziez, & Luxon, 2007; W. Kimberling, personal communication, 2009).
| Clinical Type | Subtype | Gene/Protein | Hearing Loss | Vestibular | Night Blindness Onset |
|---|---|---|---|---|---|
| I | Ib | MYO7A | Profound | Absent | 1st decade |
| Ic | Harmonin | Profound | Absent | 1st decade | |
| Id | CDH23 | Profound | Absent | 1st decade | |
| If | PCDH15 | Profound | Absent | 1st decade | |
| Ig | SANS | Profound | Absent | 1st decade | |
| II | IIa | Usherin | Moderate/ Progressive? | Normal | 2nd decade |
| IIc | VLGR1 | Sloping Moderate | Normal | 2nd decade | |
| IId | Whirlin | Sloping Moderate | Normal | 2nd decade | |
| III | IIIc | Clarin-1 | Progressive | Variable | 2nd decade |
Type I Usher syndrome presents with congenital, profound sensorineural loss (see Figure 2A) and no vestibular function. Due to the severity of the hearing loss, hearing aids are generally ineffective. These individuals are typically identified as deaf during childhood and, prior to cochlear implantation, placed in deaf education, many using sign language. Due to the vestibular areflexia, these individuals demonstrate developmental delays in sitting and walking, the latter being reported at >18 months of age (Moller, Kimberling, Davenport, Priluck, & White, 1989). Night blindness is typically apparent by age 10, so these children may be afraid of the dark and are often described as clumsy because they bump into, or trip over, objects. Significant deterioration of visual field and acuity begins between the second and third decade of life, with cataracts being a common complication (Edwards, Fishman, Anderson, Grove, & Derlackie, 1998; Piazza, Fishman, Farer, Derlacki, & Anderson, 1986; A. Sadeghi, Eriksson, Kimberling, Sjostrom, & Moller, 2006). Because the vision impairment is typically discovered later, the family may experience a second grief cycle upon learning that their deaf child will eventually be blind.
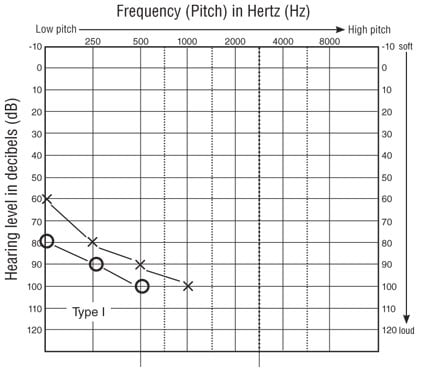
Figure 2A. Typical audiometric findings in Type I Usher syndrome.
Type II Usher syndrome exhibits a stable, sloping, moderate-to-severe sensorineural hearing loss (see Figure 2B) and typically responds well to wearable amplification. Vestibular function is normal, and visual difficulties may not be apparent until the second decade of life. Again, the prognosis of eventual blindness comes as a shock. Many patients with Usher Type II report a sense of progressive hearing loss, but Reisser, Kimberling, and Otterstedde (2002) found no evidence of progression and postulated that the phenomenon was likely due to patients' increasing loss of vision. According to this theory, as patients lose more visual cues for communication they perceive their hearing to be worse. The subtype IIa, however, may demonstrate progressive loss not found in other Type II expressions (M. Sadeghi et al., 2004). While the sequence of RP progression is similar to that of other types of RP (Innaccone et al., 2004), Type II acuity and visual field impairments are somewhat less severe compared with Type I during the third and fourth decades of life (Edwards et al., 1998; Piazza et al., 1986).
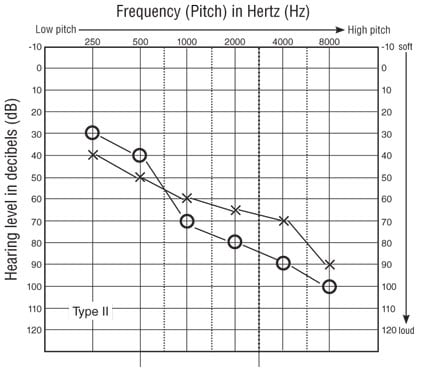
Figure 2B. Typical audiometric findings in Type II Usher syndrome.
Type III Usher syndrome presents much like Type II but with progressive hearing loss and variable vestibular function. During the first decade of life, the hearing loss is moderate sloping to profound and progresses to profound by the fourth decade (M. Sadeghi, Cohn, Kimberling, Tranebjarg, & Moller, 2005). This clinical type, while common in Finland (Plantinga et al., 2006), is rare in the United States.
It is important for the professional to understand these are general clinical types. There are some atypical expressions of Usher syndrome, and our knowledge of the underlying molecular processes is evolving (see Cohen et al., 2007, for a review). In addition, as time progresses, the differences between the clinical types can become blurred. For example, hearing loss may progress due to environmental factors and/or presbycusis, and in spite of differing ages of onset, the vision loss by the fifth decade begins to look similar across the various types (Edwards et al., 1998). This variability of expression and progression can confound the use of the clinical types, so without definitive genetic results, the clinician should proceed with caution to avoid misdiagnosis.
How Prevalent Is Usher Syndrome?
The prevalence of Usher syndrome is routinely reported as 3% to 6% of all deaf and hard of hearing individuals (Boughman, Vernon, & Shaver, 1983). The number of students with Usher syndrome attending the National Technical Institute of Deaf (NTID) between 1995 and 2005 varied from 2% to 6% (Wallber, 1995–2005). As with the NTID figure, prevalence studies have focused on the deaf population, largely excluding the hard of hearing population. Some researchers believe the true prevalence of Usher syndrome is closer to 17% when considering the total deaf and hard of hearing population (W. Kimberling, personal communication, 2009).
What Is RP?
The vision loss associated with Usher syndrome is caused by RP, a degenerative disease of the retina leading to progressive vision loss and eventual blindness. Individuals are born sighted and gradually lose their vision in a predictable manner; however, the onset and progression rate are highly variable. It is this delayed onset of expression that presents a challenge to the audiologist in assisting families with the diagnosis of Usher syndrome.
RP results from a genetic malformation or absence of one or more vital proteins for retinal survival. This is why children with Usher syndrome see quite normally at first but experience diminished vision over time. The photoreceptors gradually die, with the rods being particularly susceptible. This explains why the first symptom of RP is night blindness followed by loss of peripheral vision (tunnel vision). Only later do cones become involved, further restricting the visual field and disrupting perception of fine detail and color. Many persons with Usher syndrome, however, maintain a small pinhole of usable vision well into midlife. In fact, 51% of individuals with Type I and 72% with Type II maintain visual acuity of 20/40 or better in a small visual field in at least one eye (Edwards et al., 1998).
How Is Blindness Defined?
The definition of legal blindness includes two visual parameters: visual acuity and visual field. A person with visual acuity equal to or greater than 20/200 and/or a visual field of 20 degrees or less is legally blind. Obviously, there are many persons with low vision who do not meet these criteria, and persons with Usher syndrome may live many years before becoming legally blind. While individual states set regulations for driving licensure, an unrestricted license generally requires visual acuity of 20/40 or better and a visual field of 170° horizontally in at least one eye. Restricted licenses are available for limited situations (e.g., daylight only) for some individuals with other levels of visual competence. Given this, many individuals with Usher syndrome try driving for some time in their lives but are always faced with giving it up at some point.
How Is Usher Syndrome Diagnosed?
Behavioral and objective measures of the auditory system are familiar territory for the audiologist. Just as with hearing measures, the evaluation of visual function can be behavioral (i.e., acuity and visual field) or objective. Objective testing includes direct examination of the retina, where an ophthalmologist will find attenuated blood vessels, a waxy pallor, and clumps of dead retinal cells called bone spicules (Carr & Noble, 1981). However, these physical findings are not obvious until well after the patient is symptomatic. The definitive test of RP is an electroretinogram (ERG). Like an auditory brainstem response, an ERG is an evoked response but from the rods and cones of the retina. Because the test requires insertion of a contact lens/electrode array, general anesthesia is required with young children while topical medications can be used with adults. Although a child may still have relatively good vision, the ERG will be reduced or absent when RP/Usher syndrome is present. There is some evidence that while amplitude loss of the ERG is similar between Usher Type I and Type II, the implicit time delay may differentiate between the types (Seeliger, Zrenner, Apfelstedt-Sylla, & Jaissle, 2001). An ERG presents a distinct diagnostic advantage in that it will be abnormal long before physical signs of cell death (bone spicules) appear on the retina. This explains why an ophthalmologist often misses the diagnosis with a young patient, as the child will perform well on the visual acuity test and his or her retinas appear normal under direct examination.
Genetic testing is becoming more widely available but can be costly. Some researchers advocate that when a child is identified as deaf, testing for the GJB2 gene defect (connexin 26) would identify 40% of the population's etiology, leaving 60% to then be tested for Usher syndrome (W. Kimberling, personal communication, 2009). Once a child is diagnosed with Usher syndrome, there is genetic testing available to determine the genetic type. Centers like the Boys Town National Research Hospital Center for the Study and Treatment of Usher Syndrome in Omaha, NE, offer testing as part of their ongoing research. Many patients ask what the benefit of such testing is for them, as there is no treatment or cure for the disorder. Of course, the primary benefit is their contribution to the collective body of research on the genetic mechanisms of Usher syndrome and potential treatments. In addition, an individual may want to be genetically typed and put on a list for potential treatment trials.
What Treatment Is Available for Usher Syndrome?
There is currently no medical treatment to prevent, slow the progression of, or inhibit the transmission of Usher syndrome. Cochlear implantation is a viable, proven option for individuals with Usher syndrome. Individuals with Type I are candidates from birth, while those with Type II or III may become candidates over time. Significant quality of life improvements have been demonstrated for children with congenital deafness who receive cochlear implants (Schorr, Roth, & Fox, 2009), and implantation has been demonstrated as beneficial for deaf children with a variety of associated disabilities (Berrettini et al., 2008). Because the hearing loss in Usher syndrome is cochlear in nature, cochlear implantation is a reasonable recommendation and has, in fact, been shown to benefit auditory and social functioning of children with Usher syndrome Type I (Damon, Pennings, Snik, & Mylanus, 2006; Liu et al., 2008).
To date there is no treatment for RP. Many individuals do maintain a small, usable visual field well into the fifth or sixth decade of life. There is preliminary evidence that environmental and dietary factors may enhance the longevity of usable vision. These include protecting the retina from ultraviolet light by wearing 100% UVA/UVB sunglasses and maintaining optimal overall health. Exercise and diet, such as including seafood rich in omega-3, have also been suggested (Berson, 2000). Studies on vitamin A and docosahexaenoic acid (DHA) treatments have created some controversy (Massof & Finkelstein, 1993) and to date have only been conducted on hearing persons with nonsyndromic RP and some Usher Type II patients (Berson et al., 1993, 2004a, 2004b). Patients should be cautioned concerning vitamin A supplementation because consuming too much can cause hypervitaminosis resulting in blindness, lack of growth, and even death. Pregnant women and women who may become pregnant should not use supplements such as vitamin A because they can cause birth defects in the fetus.
Psychosocial Implications of Usher Syndrome
The dual sensory loss, particularly the progressive loss of vision, presents multiple challenges for individuals with Usher syndrome and their families. Typically the hearing loss has been addressed and decisions have been made about communication methodology. A Deaf culture identity may have been established before the knowledge of the impending vision loss. Parents are often overwhelmed by the second diagnosis and believe they should protect their child from this information until later. Meanwhile, the child may suspect something and become even more frightened because he or she does not understand. Sharing age-appropriate information with children is the best course. They do not need to be told they are "going blind," but they do need to know that they see differently and that there are strategies that will help them navigate their world. Enlisting the help of professional counseling may be warranted. Living with Usher syndrome requires lifelong adaptations to changing vision status (Miner, 1995) and in some cases, changing hearing abilities. As audiologists, we cannot assume there are professionals familiar with the impact of dual sensory loss. Most vision specialists do not know about hearing loss and in fact depend on audition to assist their blind clients. Individuals with Usher syndrome are always deaf or hard of hearing before they are visually impaired or blind. For an overview of intervention strategies, please refer to the Appendix.
The Audiologist's Role in Review
The diagnosis of significant hearing loss in a child can so dominate our attention as audiologists that we may overlook other disabling conditions. Parents rely on audiologists for information and support in working with their children who have hearing loss. Indeed, Steinberg and colleagues (2007) identified parental interactions with the audiologist as a major theme in their examination of parental narratives concerning genetic testing for hearing loss. The authors indicated that parental expectations of audiologists include information regarding resources, support and guidance through referrals and testing, and enabling the parents to adapt to their new roles. In summary, "audiologists are often expected to counsel, guide and help parents through their decisions and choices" (Steinberg et al., 2007, p. 64). In addition, the American Speech-Language-Hearing Association (ASHA) Guidelines for the Audiologic Assessment of Children From Birth to 5 Years of Age recommend that audiologists should "as appropriate, discuss additional specialty evaluations (e.g., genetics, ophthalmology, child development) with parents/caregivers and the infants' primary care provider" (ASHA, 2004, p. 19). This requires that the audiologist be familiar with the genetic epidemiology of hearing loss and resources for referral and information.
Other Forms of Combined Hearing and Vision Loss
According to the Gallaudet Research Institute (2008), 40% of all children with sensorineural hearing loss are known to have one or more additional disabling conditions. Among this group, 10% have uncorrectable vision impairment. Differentiating between children who are visually impaired and those who are deaf-blind is fraught with difficulties due to the variability in residual hearing and vision function. Vision loss, like hearing loss, varies greatly in severity and can be measured as visual near and far acuity, peripheral field, contrast sensitivity, color vision, glare sensitivity, and/or night vision. Just as you cannot truly predict a patient's communicative competency from a pure-tone audiogram, a single visual parameter does not predict visual functioning.
While Usher is the syndrome most frequently associated with RP, there are other conditions the clinician should be familiar with. Alport syndrome manifests with hearing loss, progressive nephritis, and macular flecks that may be misinterpreted as RP, while Refsum disease presents with anosmia, deafness, and true RP (Hamel, 2006). Other conditions such as CHARGE syndrome, cytomegalovirus, toxoplasmosis, and meningitis may result in combined hearing and vision loss. The 2008 National Child Count of Children and Youth Who Are Deaf-Blind reports 10,766 children ages birth through 21 years have been identified as deaf-blind (National Consortium on Deaf-Blindness, 2008). Regardless of the etiology, an audiologist needs to be sensitive to vision, and the strategies suggested here for working with patients with Usher syndrome are applicable to many other patients with compromised visual abilities.
Appendix: Intervention Strategies
Intervention for this population requires a multidisciplinary team that includes the audiologist, ophthalmologist, low vision specialist, speech-language pathologist, and educators. In addition, services from vocational rehabilitation, psychosocial counseling, genetic consultation, and orientation and mobility therapists may be necessary at different times throughout an individual's lifetime. As audiologists are often the first and primary health care provider for individuals with Usher syndrome, it behooves us to recognize and refer for proper and timely diagnosis those individuals who present with clinical signs and symptoms that may suggest the presence of RP.
At School
The following section suggests possible modifications and strategies that may assist with student access and success in the classroom. Vision is highly individual—even students with the same diagnosis will have different needs. The following are suggestions for teachers and administrators to try at school:
Making Sign Communication Accessible
- Reduce sign space.
- Wear darker/lighter clothing (depending on skin color).
- Reduce unnecessary movement while teaching.
- Repeat comments by other students.
- Ensure adequate lighting.
- Enhance residual hearing.
- Remove sources of background distraction and glare.
- Employ a copy signer in a busy classroom with signing students .
- Employ tracking and/or tactile techniques when needed.
Making Projected Materials Accessible
- Make materials available for student to review a second time.
- Adjust classroom lighting so student can still see you during projection.
- Enlarge images.
- Use yellow transparencies with overheads to increase contrast.
- Provide student with hard copies to follow.
- Use an individual monitor with a splitter in the classroom.
- Print materials for use in visualizer on yellow paper.
Making Computers Accessible
- Provide student with cursor locator or enlarged mouse (use customizing options on computer and/or adaptive software).
- Adjust monitor brightness and color for best contrast.
- Use larger font size and/or enlarging software.
- Apply keycap enlargers when needed.
- Tape a yellow transparency over the screen to decrease glare and increase contrast.
Making Printed Materials Accessible
- Use nonglare papers.
- Provide clear copies (laser print quality).
- Enlarge when needed.
- Putting a yellow transparency over pages will increase contrast for reading.
- Employ closed-circuit television (CCTV).
- Type comments on student papers rather than writing them.
- Use dark ink (never red) when writing on papers.
- Allow student to write answers instead of using computer score sheets.
- Employ tactile enhancement.
Making Black/Whiteboards Accessible
- Describe what you are writing.
- Use black markers on white boards (avoid colors).
- Use yellow chalk on clean blackboards (avoid colors).
- Use combined upper- and lowercase.
- Give student hard copies ahead of time.
- Stay close to the images on the board.
General Considerations at School
- Assign buddies so the student with Usher syndrome has someone to serve as a guide.
- Plan and practice evacuation drills.
- Allow the student time to visit and orient to new buildings before entering school there.
- Consider advance dismissal so the student can navigate hallways before they are too crowded between classes.
- Alert the student to changes in furniture, schedules, and any construction.
- Make it a policy that doors are always closed, never left half open (so the student won't run into the door).
School administrators, teachers, and counselors should be referred to Hicks & Hicks (1981), Miner (1995), and Chen (2004) for information on working in educational settings with children who have Usher syndrome.
For Parents
- Do share age-appropriate information. Younger children are more resilient and can adapt, so don't wait until adolescence when they have so many other challenges. They often know something is wrong anyway and without information may be more frightened than necessary.
- Teach your child to carry a flashlight. Be sure your child knows his or her way around the neighborhood and understands daylight changes over time.
- Children with Usher Type I may have significant difficulty riding wheeled objects (bicycles, skateboards, etc.) and need close supervision in water. Once submerged, they may become confused as to which direction is the water surface.
- Teach older children to use public transportation. This is a skill they will need throughout life because they will be unable to drive an automobile.
- Introduce mobility skills early before stigma is associated with such things.
- Find adult role models with Usher syndrome so your child (and you) can envision a future.
- Ensure adequate lighting in your home.
About the Author
Josara Wallber, AuD, CCC-A, spent 25 years at the National Technical Institute for the Deaf, where she taught in the deaf education program and was a certified ophthalmic assistant working closely with the deaf-blind community. She also worked with aural rehabilitation, amplification, and cochlear implants for college students. She coproduced the documentary "Silence With a Touch: Living With Usher Syndrome," which received an Award of Excellence from Communicator Awards in 2007. She is now an associate clinical professor at Idaho State University, where she teaches and supervises clinical activities with an emphasis on cochlear implants. Contact her at walljosa@isu.edu.
References
American Speech-Language-Hearing Association. (2004). Guidelines for the audiologic assessment of children from birth to 5 years of age. Available from www.asha.org/policy/.
Berrettini, S., Forli, F., Genovese, E., Santarello, R., Arslan, E., Chilosi, A., & Cipriani, P. (2008). Cochlear implantation in deaf children with associated disabilities: Challenges and outcomes. International Journal of Audiology, 47, 199–208.
Berson, E. (2000). Nutrition and retinal degeneration. International Ophthalmology Clinics, 40(4), 93–111.
Berson, E., Rosner, B., Sandberg, M., Hayes, K., Nicholson, B., Weigel-DiFranco, C., & Willett, W. (1993). A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Archives of Ophthalmology, 111, 761–772.
Berson, E., Rosner, B., Sandberg, M., Weigel-DiFranco, C., Moser, A., Brockhurst, R., et al. (2004a). Clinical trial of docosahexaenoic acid in patients with retinitis pigmentosa receiving vitamin A treatment. Archives of Ophthalmology, 122, 1297–1305.
Berson, E., Rosner, B., Sandberg, M., Weigel-DiFranco, C., Moser, A., Brockhurst, R., et al. (2004b). Further evaluation of docosahexaenoic acid in patients with retinitis pigmentosa receiving vitamin A treatment: Subgroup analyses. Archives of Ophthalmology, 122, 1306–1314.
Boughman, J., Vernon, M., & Shaver, K. (1983). Usher syndrome: Definition and estimate of prevalence from two high-risk populations. Journal of Chronic Diseases, 36, 595–603.
Carr, R., & Noble, K. (1981). Retinitis pigmentosa. Ophthalmology, 88(2), 169–172.
Chen, D. (2004). Young children who are deaf-blind: Implications for professional in deaf and hard of hearing services. Volta Review, 104(4), 273–284.
Cohen, M., Bitner-Glindziez, M., & Luxon, L. (2007). The changing face of Usher syndrome: Clinical implications. International Journal of Audiology, 46, 82–93.
Damon, G., Pennings, R., Snik, A., & Mylanus, E. (2006). Quality of life and cochlear implantation in Usher syndrome type I. Laryngoscope, 116, 723–728.
Edwards, A., Fishman, G., Anderson, R., Grove, S., & Derlackie, D. (1998). Visual acuity and visual field impairment in Usher syndrome. Archives of Ophthalmology,116, 165–168.
Gallaudet Research Institute. (2008). Regional and national summary report of data from the 2007–08 annual survey of deaf and hard of hearing children and youth. Washington, DC: Author.
Gorlin, R., Torella, H., & Cohen, M. (1995). Hereditary hearing loss and its syndromes. Oxford, England: Oxford University Press.
Hamel, C. (2006). Retinitis pigmentosa. Orphanet Journal of Rare Diseases, 1(40), 1–12.
Harrison, S., & Rousch, J. (1996). Age of suspicion, identification, and intervention for infants and young children with hearing loss: A national study. Ear and Hearing, 17, 55–62.
Hicks, W., & Hicks, D. (1981). The Usher's syndrome adolescent: Programming implications for school administrators, teachers, and residential advisors. American Annals of the Deaf, 26, 422–431.
Innaccone, A., Kritchevsky, S., Ciccarelli, M., Tedesco, S., Macahuso, C., Kimberling, W., & Somes, G. (2004). Kinetics of visual field loss in Usher syndrome type II. Investigative Ophthalmology & Visual Science, 45, 784–792.
Keats, B. (2002). Genes and syndromic hearing loss. Journal of Communication Disorders, 33, 355–366.
Kimberling, W., & Lindenmuth, A. (2007). Genetics, hereditary hearing loss, and ethics. Seminars in Hearing, 28(3), 216–225.
Liu, X., Angeli, S., Rajput, K., Yan, D., Hodges, A., Eshraghi, A., et al. (2008). Cochlear implantation in individuals with Usher type 1 syndrome. International Journal of Pediatric Otorhinolaryngology, 72, 841–847.
Massof, R., & Finkelstein, D. (1993). Supplemental vitamin A retards loss of ERG amplitude in retinitis pigmentosa. Archives of Ophthalmology,111, 751–754.
Miner, I. (1995). Psychosocial implications of Usher syndrome, type I, throughout the life cycle. Journal of Visual Impairment & Blindness, 89(3), 287–296.
Moller, C., Kimberling, W., Davenport, S., Priluck, L., & White, V. (1989). Usher syndrome: An otoneurologic study. Laryngoscope, 99, 73–79.
National Consortium on Deaf-Blindness. (2008). National Child Count of Children and YouthWho Are Deaf-Blind. Retrieved December 2, 2009, from www.nationaldb.org/documents/products/2008-Census-Tables.pdf [PDF].
Piazza, L., Fishman, G., Farer, M., Derlacki, D., & Anderson, R. (1986). Visual acuity loss in patients with Usher's syndrome. Archives of Ophthalmology, 104, 1336–1339.
Plantinga, R., Pennings, R., Huygen, P., Sankila, E., Tuppurainen, K., Kleemola, L., et al. (2006). Visual impairment in Finnish Usher syndrome type III. Acta Ophthalmologica Scandinavica, 84(1), 36–41.
Reisser, C., Kimberling, W., & Otterstedde, C. (2002). Hearing loss in Usher syndrome type II is nonprogressive. Annals of Otology, Rhinology and Laryngology, 111, 1108–1111.
Sadeghi, A., Eriksson, K., Kimberling, W., Sjostrom, A., & Moller, C. (2006). Longterm visual prognosis in Usher syndrome types 1 and 2. Acta Ophthalmologica Scandinavica, 84, 537–544.
Sadeghi, M., Cohn, E., Kelly, W., Kimberling, W., Tranebjarg, L., & Moller, C. (2004). Audiological findings in Usher syndrome types IIa and II (non-IIa). International Journal of Audiology, 43, 136–143.
Sadeghi, M., Cohn, E., Kimberling, W., Tranebjarg, L., & Moller, C. (2005). Audiological and vestibular features in affected subject with USH3: A genotype/phenotype correlation. International Journal of Audiology, 44, 307–316.
Schorr, E., Roth, F., & Fox, N. (2009). Quality of life for children with cochlear implants: Perceived benefits and problems and the perception of single words and emotional sounds. Journal of Speech, Language, and Hearing Research, 52, 141–152.
Seeliger, M., Zrenner, E., Apfelstedt-Sylla, E., & Jaissle, G. (2001). Identification of Usher syndrome subtypes by ERG implicit time. Investigative Ophthalmology and Visual Science, 42, 3066–3071.
Steinberg, A., Kaimal, G., Ewing, R., Soslow, L., Lewis, K., Krantz, I., & Li, Y. (2007). Parental narratives of genetic testing for hearing loss: Audiologic implications for clinical work with children and families. American Journal of Audiology, 16, 57–67.
Wagenaar, M., Snik, A., Kimberling, W., & Cremers, C. (1996). Carriers of Usher syndrome type IB: Is audiometric identification possible? The American Journal of Otology, 17, 853–858.
Wallber, J. (1995–2005). Annual reports of the Eye & Ear Clinic. Rochester, NY: National Technical Institute for the Deaf at Rochester Institute of Technology.
Resources
- Boys Town National Research Hospital Usher Syndrome webpage
- National Consortium on Deaf-Blindness
- National Consortium on Deaf-Blindness, State Deaf-Blind Projects
- National Institute on Deafness and Other Communication Disorders Usher Syndrome webpage
- National Institutes of Health Usher Syndrome webpage
- National Library of Medicine Genetics Home Reference
- Second Sight Medical Products, Inc. retinal prosthesis—investigational device
- Understanding Usher Syndrome: Information for School Counselors [PDF]











