Monitoring Ototoxicity in the Pediatric Oncology Population
June 2013
Johnnie K. Bass, AuD, CCC-A; Stephanie T. White, AuD, CCC-A; Skye E. Jones, AuD, CCC-A
Introduction
While the overall incident rate for cancer increased slightly in children ages 14 years between 2005 and 2009, the death rate decreased by more than half and the 5-year survival rate for many pediatric cancers in the United States has reached upwards of 80% or more (Siegel, Naishadhamc, & Jemal, 2013). Advancements in the diagnosis and treatment of childhood cancers have led to these increased long-term survival rates. Unfortunately, a number of adverse side effects, including hearing loss, continue to be a concern for all involved. Hearing loss is a potential adverse effect of platinum-based chemotherapeutic agents (cisplatin and carboplatin; McHaney, Thibadoux, Hayes, & Green, 1983; Qaddoumi et al., 2012) and cranial radiation involving the auditory structures (Ho et al., 1999; Hua, Bass, Khan, Kun, & Merchant, 2008).
Hearing loss acquired at a young age can significantly delay speech and language development (Yoshinaga-Itano, Sedey, Coulter, & Mehl, 1998) and can negatively impact educational outcomes and psychosocial development (Knight, Kraemer, & Neuwelt, 2005). Early identification and intervention of hearing loss in young children is critical for optimizing normal speech and language development, academic achievement, and psychosocial well-being (Downs & Yoshinaga-Itano, 1999; Yoshinago-Itano, 1999; Yoshinaga-Itano et al., 1998). Ototoxicity monitoring before, during, and after treatment is an important component in the early detection and management of hearing loss in young cancer patients.
This article provides a brief overview of potentially ototoxic agents routinely prescribed to treat cancer in children. A case study approach using fictional audiological test results representative of actual cases seen in our clinic will be used to describe various types of hearing loss caused by platinum-based chemotherapeutic agents and cranial radiation therapy. Additionally, the St. Jude ototoxicity monitoring protocol for pediatric patients receiving cisplatin, carboplatin, and/or cranial radiation therapy is outlined.
Platinum-Induced Ototoxicity
The platinum-based compounds carboplatin and cisplatin are effective chemotherapy agents commonly used to treat a variety of childhood cancers. Unfortunately, the use of these drugs can lead to significant permanent hearing loss (Jehanne et al., 2009; Li, Womer, & Silber, 2004; McHaney et al., 1983; Qaddoumi et al., 2012). While carboplatin is far less ototoxic than cisplatin, the risk for developing hearing loss from this drug should not be discounted (Jehanne et al., 2009; Parsons et al., 1998; Qaddoumi et al., 2012). Platinum-induced hearing loss is typically bilateral, symmetrical, and sensorineural in nature (Blakley & Myers, 1993; Higby, Qaddoumi, et al., 2012; Wallace, Albert, & Holland, 1974; Waters, Ahmad, Katsarkas, Stanimir, & McKay, 1991). High-frequency hearing is affected first, with hearing loss eventually progressing to the lower frequencies with increasing cumulative doses (Li et al., 2004). Cumulative cisplatin doses exceeding 400 mg/m 2(Li et al., 2004) and carboplatin administered in high, myeloablative doses (Parsons et al., 1998) have been shown to increase the risk of hearing loss, with younger age at exposure further increasing the risk of ototoxicity of these drugs (Li et al., 2004; Qaddoumi et al., 2012). Li et al. (2004) found that children younger than age 5 were 21 times more likely to develop moderately-severe high frequency hearing loss after exposure to cisplatin than older children and adolescents given the same cumulative dose (400 mg/m 2). Qaddoumi and colleagues (2012) demonstrated that infants treated with carboplatin at less than 6 months of age were at greater risk for developing ototoxicity compared with older children treated similarly.
Platinum-induced ototoxicity typically occurs during or shortly after treatment cessation. However, late onset of hearing loss (i.e., hearing loss occurring months to years after therapy) has been documented in patients treated with cisplatin (Kolinsky, Hayaski, Karzon, Mao, & Hayaski, 2010) and carboplatin (Jehanne et al., 2009; Qaddoumi et al., 2012). Hearing loss can continue to worsen years following therapy cessation in patients treated with cisplatin (Bertolini et al., 2004; Einarsson et al., 2010). For these reasons, long-term follow-up is essential in pediatric patients exposed to platinum chemotherapy.
The following case studies are fictitious but represent typical treatment regimens and hearing losses seen in our clinic.
Cisplatin Case Study
This young patient was treated with three cycles of high dose cisplatin chemotherapy (600 mg/m2) at less than 3 years of age. A baseline evaluation (conducted prior to starting therapy) via ABR/ASSR (dBeHL) assessment revealed normal hearing sensitivity, bilaterally (Figure 1a). An ABR/ASSR (dBeHL) assessment completed after the second cycle of cisplatin chemotherapy indicated normal hearing sensitivity through 2000 Hz, sloping to a severe high-frequency hearing loss, bilaterally (Figure 1b). At the conclusion of treatment, conditioned play audiometry revealed normal hearing sensitivity at 500 Hz, sloping from a mild to severe hearing loss at 1000-8000 Hz, bilaterally (Figure 1c). This hearing loss example is typical of high dose cisplatin treatment, particularly in children diagnosed and treated at less than 5 years of age.

Figure 1a. Baseline ABR/ASSR (eHL) evaluation

Figure 1b. ABR/ASSR (eHL) evaluation post 2 cycles of cisplatin

Figure 1c. Conditioned play audiometry following 3 cycles of high dose cisplatin
Carboplatin Case Study
This patient was diagnosed and treated as an infant with six courses of carboplatin chemotherapy. As seen in Figure 2a, the patient's baseline evaluation via ABR/ASSR (dBeHL) assessment revealed normal hearing sensitivity, bilaterally. An ABR/ASSR evaluation completed following six cycles of carboplatin chemotherapy indicated normal hearing sensitivity at 1000-4000 Hz, sloping to a severe bilateral high-frequency hearing loss (Figure 2b). Two years post diagnosis, an ABR/ASSR (dBeHL) evaluation and VRA assessment confirmed normal hearing sensitivity at 1000 Hz, sloping from a mild to severe bilateral high-frequency hearing loss (Figure 2c). This example demonstrates the increased risk of ototoxicity being present at a young age (<6 months) and the delayed onset of hearing loss sometimes seen in patients receiving carboplatin.
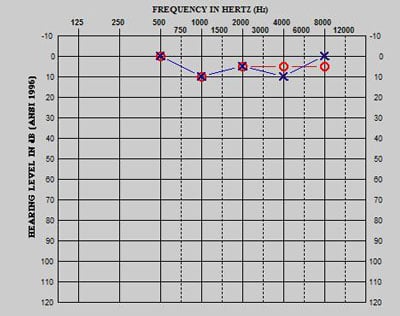
Figure 2a. Baseline ABR/ASSR (eHL)
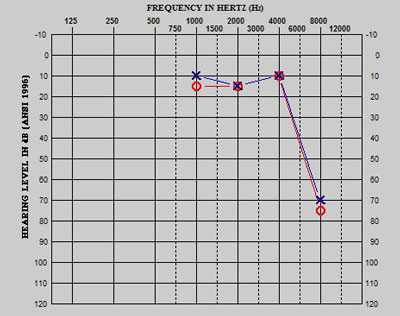
Figure 2b. End of therapy ABR/ASSR (eHL) evaluation post 6 cycles

Figure 2c. Progressive, delayed onset of serious hearing loss 2 years post treatment
Radiation-Induced Ototoxicity
Cranial radiation therapy is routinely used to treat a variety of childhood brain tumors and head and neck cancers. Hearing loss is a potential adverse consequence of this therapy, particularly when the hearing mechanism is located within the radiation field (Ho et al., 1999; Hua et al., 2008). Cranial radiation can potentially damage any of the auditory structures extending from the external ear to the higher auditory pathways. Thus, hearing loss can present as conductive, mixed, sensorineural, or retrocochlear in nature (Jereczek-Fossa, Jarowski, Milani, & Orecchia, 2003).
Radiation therapy can degrade the external ear and middle ear system, causing a temporary or permanent conductive hearing loss. Conductive hearing loss can be the result of skin reactions, otitis externa, cerumen occlusion, chronic eustachian tube dysfunction, and middle ear effusion. Documented skin reactions can include erythema, dry and moist desquamation, ulceration, atrophy, external canal stenosis, and otitis externa (Jereczek-Fossa et al., 2003; Johannesen, Rasmussen, Winther, Halvorsen, & Lote, 2002). Acute complications to the middle ear system, resulting in otitis media and eustachian tube dysfunction, have been documented in 40% of patients treated with radiation therapy (Jereczek-Fossa et al., 2003).
A reported one-third of patients treated with cranial radiation involving the cochlea experience sensorineural hearing loss (Jereczek-Fossa et al., 2003). Radiation and hearing loss exhibit a dose-response relationship—as radiation dose increases, so does risk for hearing loss and degree of severity (Bhandare et al., 2010; Hua et al., 2008). Hua and colleagues (2008) demonstrated that pediatric patients did not experience hearing loss when the mean cochlear radiation dose was less than 35 Gy. However, risk for hearing loss significantly increased when the mean cochlear radiation dose rose above 35 Gy (Hua et al., 2008). Cranial radiation has also been shown to exacerbate hearing loss when administered with concurrent or adjuvant platinum-based chemotherapies (Low, Toh, Wee, Fook-Chong, & Wang, 2006).
Sensorineural hearing loss from radiation exposure affects the high frequencies more severely than the low frequencies (Fong, Beste, & Murray, 1995; Hua et al., 2008) and is typically irreversible and progressive in nature (Ho et al., 1999). Radiation-induced hearing loss is typically considered a late adverse effect with onset occurring several years after treatment (Ho et al., 1999; Hua et al., 2008; Low et al., 2006; Williams, Kun, Thompson, Gould, & Stocks, 2005). Thus, long-term audiological follow-up is recommended for patients considered high risk (cochlear exposure >35 Gy) for radiation-induced hearing loss.
Cranial Radiation Case Study
This patient was diagnosed with a brain tumor at less than 3 years of age. Treatment included a gross total resection and focal irradiation of 59.4 Gy. The left cochlea was exposed to 43.0 Gy, and the right cochlea was exposed to 59.4 Gy. As shown in Figure 3a, a baseline audiological evaluation prior to cranial radiation revealed normal hearing sensitivity, bilaterally. Audiological testing 2 years post treatment indicated normal hearing sensitivity, bilaterally (Figure 3b). Re-evaluation 3 years post treatment revealed significant bilateral hearing loss (Figure 3c). This case represents a patient who was exposed to radiation levels above 35 Gy but did not experience hearing loss until approximately 3 years after treatment. While high frequencies are typically affected initially, some patients present with atypical hearing loss configuration as evident in this example.

Figure 3a. Baseline evaluation
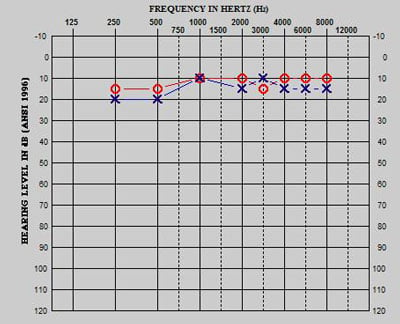
Figure 3b. Evaluation 2 years post therapy
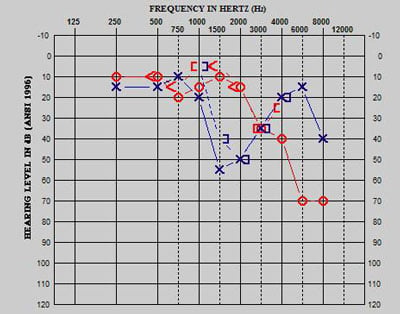
Figure 3c. Evaluation 3 years post therapy
St. Jude Children's Research Hospital Ototoxicity Monitoring Protocol
Figure 4 [PDF] outlines a general ototoxicity monitoring protocol followed at St. Jude Children's Research Hospital. This protocol was adapted from the ototoxicity monitoring guidelines issued by the American Speech-Language-Hearing Association (1994) and the American Academy of Audiology (2009). This protocol serves as a guideline only (Figure 4 [PDF], Ototoxicity monitoring protocol for platinum chemotherapy and cranial radiation therapy).
Often, clinical trials and hospital-driven treatment protocols have specific time points for performing audiological assessments. These time points are predicted based on several factors, such as diagnosis, treatment plan, patient age, and ototoxicity risk. Of course, the patient should be evaluated at any time during or after therapy if a noticeable change in hearing occurs. Patients diagnosed with severe or fluctuating hearing loss should be followed more frequently than the ototoxicity protocol indicates, at least until hearing loss stabilizes.
Managing High Frequency Hearing Loss in Children
While carboplatin is substantially less ototoxic than cisplatin, both can lead to serious hearing loss requiring the use of assistive listening technology, preferential classroom seating, additional educational resources, and hearing aids. Managing pediatric patients with significant high-frequency hearing loss typically involves patient/parent counseling, including the importance of avoiding loud noise exposure, use of hearing protection, and use of communication strategies such as preferential seating. Patients with serious high-frequency hearing loss typically benefit from hearing aids and assistive listening devices. Hearing aids that offer open fitting options and frequency compression technology have improved hearing aid acceptance and long-term use in our patient population. At this time, cochlear implantation for this population is rare, as hearing loss is typically contained in the high-frequency range, sparing the low frequencies. Occasionally, some patients experience progressive hearing loss, creating the need for cochlear implantation.
Managing hearing loss in patients who receive radiation therapy for head and neck tumors can be complex. In those cases resulting in chronic conductive hearing loss, management recommendations are usually straightforward and include conventional hearing aids, bone conduction hearing aids, and/or assistive listening devices. These recommendations are based on the extent of the conductive hearing loss and are made in collaboration with an ENT physician. Managing patients diagnosed with radiation-induced sensorineural hearing loss can be more challenging. Generally, hearing aids and assistive listening devices are recommended for these patients. However, it has been our experience that many patients with sensorineural hearing loss from radiation exposure exhibit significantly poor speech understanding, typically poorer than would be expected in the presence of better pure tone thresholds. Thus, the patient may not perceive benefit from conventional hearing aids and ultimately reject amplification. These patients are treated on a case-by-case basis. Trial use of other options, such as FM technology or CROS hearing devices, is often recommended and can be successful, particularly for patients with asymmetrical hearing loss. Ongoing counseling and coordination of services with educational audiologists, specialists with hearing impairment, speech-language pathologists, and other professionals are important in helping patients return to the classroom and receive the appropriate resources they need to succeed.
Conclusion
Pediatric oncology patients treated with platinum agents or cranial radiation are at risk for developing significant hearing loss. Audiologists have a unique opportunity to implement an ototoxicity monitoring protocol for these patients and to promote early intervention (i.e. chemotherapy reduction or treatment modification) when possible. In cases when cancer treatment cannot be altered, audiologists play an integral part in providing counseling, aural rehabilitation, and coordination of services in educational settings. Clearly, ototoxicity monitoring should not conclude at the end of therapy. Delayed onset and progressive hearing loss have been documented in patients treated with platinum-based chemotherapy and cranial radiation therapy. For these reasons, long-term monitoring is imperative to ensuring proper management of patients exposed to ototoxic agents.
About the Authors
Johnnie K. Bass is a research audiologist at St. Jude Children's Research Hospital. She participates in direct patient care, mainly ototoxic monitoring and aural rehabilitation in the pediatric oncology population. She is active in clinical research involving the effects of ototoxic agents and the efficacy of otoprotective drugs. Dr. Bass is currently pursuing a PhD in audiology at the University of Memphis. Contact her at johnnie.bass@stjude.org.
Stephanie T. White has been a pediatric audiologist at St. Jude Children's Research Hospital for 7 years. She received her doctoral degree from the University of Northern Colorado in 2006. Dr. White's research interests include the long-term effects of ototoxic medications, specifically hearing loss progression. Contact her at stephanie.white@stjude.org.
Skye E. Jones is currently a pediatric audiologist at St. Jude Children's Research Hospital. Dr. Jones completed a doctoral degree in audiology from the University of Memphis. Her audiological interests include diagnostic audiology, ototoxic monitoring, and clinical research. Contact her at skye.jones@stjude.org.
References
American Academy of Audiology. (2009). Ototoxicity monitoring [PDF].
American Speech-Language-Hearing Association. (1994). Audiologic management of individuals receiving cochleotoxic drug therapy guidelines.
Bertolini, P., Lassalle, M., Mercier, G., Raquin, M. A., Izzi, G., Corradini, N., & Hartmann, O. (2004). Platinum compound-related ototoxicity in children: long-term follow-up reveals continuous worsening of hearing loss. Journal of Pediatric Hematology/Oncology, 26(10), 649–655.
Bhandare, N., Jackson, A., Eisbruch, A., Pan, C. C., Flickinger, J. C., Antonelli, P., & Mendenhall, W. M. (2010). Radiation therapy and hearing loss. International Journal of Radiation Oncology • Biology • Physics, 76 (3 Suppl.), S50–S57.
Blakley, B. W., & Myers, S. F. (1993). Patterns of hearing loss resulting from cis-platinum therapy. Otolaryngology-Head and Neck Surgery, 109 (3 Pt. 1), 385–391.
Downs, M. P., & Yoshinaga-Itano, C. (1999). The efficacy of early identification and intervention for children with hearing impairment. Pediatric Clinics North America, 4(1), 79–87.
Einarsson, E. J., Petersen, H., Wiebe, T., Fransson, P. A., Grenner, J., Magnusson, M., & Moëll, C. (2010). Long term hearing degeneration after platinum-based chemotherapy in childhood. International Journal of Audiology, 49(10), 765–771.
Fong, R. S., Beste, D. J., & Murray, K. J. (1995). Pediatric sensorineural hearing loss after temporal bone radiation. American Journal of Otolaryngology, 16(6), 793–796.
Higby, D. J., Wallace Jr., H. J., Albert, D., & Holland, J. F. (1974). Diamminodichloroplatinum in the chemotherapy of testicular tumors. The Journal of Urology, 112(1), 100–104.
Ho, W. K., Wei, W. I., Kwong, D. L., Sham, J. S., Tai, P. T., Yuen, A. P., & Au, D. K. (1999). Long-term sensorineural hearing deficit following radiotherapy in patients suffering from nasopharyngeal carcinoma: a prospective study. Head & Neck, 21(6), 547–553.
Hua, C., Bass, J. K., Khan, R., Kun, L. E., & Merchant, T. E. (2008). Hearing loss after radiotherapy for pediatric brain tumors: effect of cochlear dose. International Journal of Radiation Oncology • Biology • Physics, 72(3), 892–899.
Jehanne, M., Lumbrose-LeRouic, L., Savignoni, A., Aerts, I., Mercier, G., Bours, D., Desjardins, L., & Doz, F. (2009). Analysis of ototoxicity in young children receiving carboplatin in the context of conservative management of unilateral or bilateral retinoblastoma. Pediatric Blood & Cancer, 52(5), 637–643.
Jereczek-Fossa, B. A., Zarowski, A., Milani, F., & Orecchia, R. (2003). Radiotherapy-induced ear toxicity. Cancer Treat Reviews, 29(5), 417–430.
Johannesen, T. B., Rasmussen, K., Winther. F. O., Halvorsen, U., & Lote, K. (2002). Late radiation effects on hearing, vestibular function, and taste in brain tumor patients. International Journal of Radiation Oncology • Biology • Physics, 53(1), 86–90.
Knight, K. R., Kraemer, D. F., & Neuwelt, E. A. (2005). Ototoxicity in children receiving platinum chemotherapy: Underestimating a commonly occurring toxicity that may influence academic and social development. Journal of Clinical Oncology, 23(34), 8588–8596.
Kolinsky, D. C., Hayashi, S. S., Karzon, R., Mao, J., & Hayashi, R. J. (2010). Late onset hearing loss: a significant complication of cancer survivors treated with cisplatin containing chemotherapy regimens. Journal of Pediatric Hematology/Oncology, 32(2), 119–123.
Li, Y., Womer, R. B., & Silber, J. H. (2004). Predicting cisplatin ototoxicity in children: the influence of age and the cumulative dose. European Journal of Cancer, 40(16), 2445–2451.
Low, W. K., Toh, S. T., Wee, J., Fook-Chong, S. M., & Wang, D. Y. (2006). Sensorineural hearing loss after radiotherapy and chemoradiotherapy: a single, blinded, randomized study. Journal of Clinical Oncology, 24(12), 1904–1909, 2006.
McHaney, V. A., Thibadoux, G., Hayes, F. A., & Green, A. A. (1983). Hearing loss in children receiving cisplatin chemotherapy. Journal of Pediatrics, 102(2), 314–317.
Parsons, S. K., Neault, M. W., Lehmann, L. E., Brennan, L. L., Eickhoff, C. E., Kretschmar, C. S., & Diller, L. R. (1998). Severe ototoxicity following carboplatin-containing conditioning regimen for autologous marrow transplantation for neuroblastoma. Bone Marrow Transplantation, 22(7), 669–674.
Qaddoumi, I., Bass, J. K., Wu, J., Billups, C. A., Wozniak, A. W., Merchant, T. E., & ... Rodriguez-Galindo, C. (2012). Carboplatin-associated ototoxicity in children with retinoblastoma. Journal of Clinical Oncology, 30(10), 1034–1041.
Siegel, R., Naishadhamc, D., & Jemal, A. (2013). Cancer statistics, 2013. CA: A Cancer Journal for Clinicians, 63(1), 11–30.
Yoshinago-Itano, C., Sedey, A. L., Coulter, D. K., & Mehl, A. L. (1998). Language of early- and later-identified children with hearing loss. Pediatrics, 102(5), 1161–1171.
Yoshinaga-Itano, C. (1999). Benefits of early intervention for children with hearing loss. Otolaryngologic Clinics of North America, 32(6), 1089–1102.
Waters, G. S., Ahmad, M., Katsarkas, A., Stanimir, G., & McKay, J. (1991). Ototoxicity due to cis-diamminedichloroplatinum in the treatment of ovarian cancer: influence of dosage and schedule of administration. Ear and Hearing, 12(2), 91–102.
Williams, G. B., Kun, L. E., Thompson, J. W., Gould, H. J., & Stocks, R. M. (2005). Hearing loss as a late complication of radiotherapy in children with brain tumors. Annals of Otology, Rhinology and Laryngology, 114(4), 328–331.











