Intellectual Disabilities and Hearing Loss
July 2013
Gilbert R. Herer, PhD, CCC-A/SLP
Introduction
Early detection of hearing loss and immediate intervention for people with intellectual disabilities (ID) are imperatives for their cognitive, social, and vocational well-being. The professional literature reports increased prevalence of hearing loss for people with ID compared to their general-population peers. It also reflects undetected hearing loss for individuals with ID, along with undertreated and unserved experiences once hearing loss is identified. The hearing health of children with Down syndrome (DS) has received considerable attention in published reports, and some studies have examined the hearing status of adults with ID in institutional settings. However, few investigations have focused on adults living and participating in the general community (i.e., their most frequent experience now in the United States and many other countries). This essay will discuss the hearing status reported for 9,961 non-institutionalized adults with ID (Herer, 2012).
Findings for Children with Down Syndrome and Adults with ID in Institutional Settings
The following brief review highlights the literature reports of hearing findings for children with DS and adults with ID residing in institutional settings. This review provides a framework for understanding the hearing status of adults with ID living in general-community environments as reported in this essay. Significant rates of conductive hearing loss (53%–88%) for children with DS are reported and attributed to the anatomic differences in the head and neck region of these children (Harigai, 1994; Hess, Rosanowski, Eysholdt, & Shuster, 2006; A. Hildmann, H. Hildmann, & Kessler, 2002; Kanamori, Witter, Brown, & Williams-Smith, 2000; Park, Wilson, Stevens, Harward, & Hohler, 2012; Szyfter & Laczkowska-Przybylska, 1995). Proactive hearing screening of children with DS is urged to achieve early detection, maximize opportunities for immediate treatment, as well as improve health and education (Barr, Dungworth, Hunter, McFarlane, & Kubba, 2011; Driscoll, Kei, Bates, & McPherson, 2003; Shott, Joseph, & Heithaus, 2001).
Other studies have discussed the hearing status of adults with ID residing in institutional settings, reporting rates of hearing loss ranging from 21% to 70%. These rates varied with age, with individuals younger than age 40 showing lower rates compared with older persons with ID; and those without DS exhibiting lower rates compared with those with DS. Further, a number of studies indicated a persistent lack of hearing loss identification for many adults with ID prior to their being study subjects. Essentially all investigations recommended regular hearing and health screenings for adults with ID (H. Evenhuis et al., 2004; H. M. Evenhuis, 1995; H. M. Evenhuis, Theunissen, Denkers, Verschuure, & Kemme, 2001; van Allan, Fung, & Jurenka, 1999; Van Buggenhout et al., 1999).
Hearing Status of Adults with ID Living in Their General Communities
Few studies of non-institutionalized adults with ID are reported in the literature. One noteworthy and comprehensive investigation (Meuwese-Jongejeugd et al., 2006) in The Netherlands of 1,598 individuals ages 18–70 found an overall prevalence rate for hearing loss of 36%. This outcome was twice that of the 16% to 17% prevalence rates reported for general-population studies in Great Britain, Italy, and Australia (Davis, 1989; Quaranta, Assennato, & Sallustio, 1996; Wilson et al., 1999). Further, a remarkable 48% of subjects with ID had not been identified with hearing loss before this study was undertaken.
The Meuwese-Jongejeugd et al. (2006) study also revealed progressively greater prevalence rates of hearing loss for each age decade from 18–30 through 61–70 in persons with ID. Those study subjects with DS showed significantly greater rates at each age decade compared with the rates for persons with ID unrelated to DS. The rates of hearing loss for participants with DS ranged progressively from 36.4% (ages 18–30) to 100% (ages 61–70). By striking contrast, the hearing loss prevalence rates for study subjects with ID not due to DS increased steadily from 7.5% (ages 18–31) to 52.3% (ages 61–70). Notably, the rates in the Meuwese-Jongejeugd et al. study for both groups with ID in each age decade far exceeded the rates reported for the general-population studies of Great Britain (Davis, 1989) and Italy (Quaranta et al., 1996). The latter prevalence rates of hearing loss ranged from 1.8% for ages 18–30 to 37.7% for ages 61–70.
Hearing and Other Ear Outcomes From a Study of 9,961 Non-institutionalized People with ID
With the above as background information, let's now review the recently reported study of hearing outcome findings of 9,961 non-institutionalized individuals with ID (Herer, 2012). These adults, 18–55 years of age, participated in sports competition events globally over the past decade sponsored by Special Olympics International. The hearing status and ear canal health of this very large sample provides informative perspectives of hearing and ear care needs of individuals with ID living within their general communities.
Study Protocol
The hearing examination protocol for this study included ear canal examinations, otoacoustic emissions (OAE) screening, pure tone screening at 25 dB for 2,000 Hz and 4,000 Hz using air-conduction earphones, and tympanometry screening. Those individuals not passing the pure tone screening received pure tone threshold testing of 1,000, 2,000, 4,000, and 8,000 Hz by air-conduction earphones; and 1,000, 2,000, and 4,000 Hz by bone-conduction receiver placed on the mastoid process behind an ear (Herer & Montgomery, 2006). All audiometers were calibrated to the appropriate American National Standards Institute standards for pure tone testing. All threshold testing was conducted in very quiet sound environments, mostly within sound booths brought to event sites. All testing was conducted by experienced audiologists, speech-language pathologists, and other health care professionals who were trained to use the investigation's protocol. This hearing examination protocol was found to be very efficient in detecting hearing loss and ear canal conditions in large groups of individuals with ID being screened/tested within short time periods.
Overall Hearing Outcomes
The outcome results for the 9,961 individuals with ID were gathered at seven Special Olympics (SO) sports events from 2004 through 2011. As seen in Table 1, there were strong similarities and some differences for outcomes among these events. For example, the overall prevalence rate of hearing loss for the seven events was 23.7%; but the rates clustered between 17% and 22.9% for five events, while rates for two events reached 32.2% and 38%. These differences may be due to different groups of individuals with ID participating at each event. Nevertheless, there is a notable difference in the overall central tendency of these data (23.7%) and that reported for the general non-ID population; that is, 1.4 times greater than the 16%–17% rate found in general-population studies of Great Britain, Italy, and Australia (Davis, 1989; Quaranta et al., 1996; Wilson et al., 1999).
Further, Table 1 reveals that the overall prevalence rate of sensorineural hearing loss (SNHL) for people with ID across the seven SO events was 12.8%, a rate greater than expected in the general population 18–55 years of age. Most of these individuals were candidates for hearing aid use. The overall prevalence rate for conductive/mixed (C/M) loss found at these events, 10.9%, was higher than expected in the general population of adults. All with C/M loss were in need of medical evaluation.
| Table 1 . Healthy Hearing Outcomes From Specific Special Olympics Events (Herer, 2012) | |||||
|---|---|---|---|---|---|
| Events | Number of athletes tested | Notable Cerumen (%) | Hearing Loss (%) | SNHL (%) | C/M loss (%) |
| 2004 Germany National Games | 755 | 53.0 | 38.0 | 21.3 | 16.7 |
| 2005 Japan International Winter Games | 893 | 36.0 | 20.5 | 3.6 | 16.9 |
| 2006 USA National Games | 753 | 39.0 | 18.0 | 11.8 | 6.2 |
| 2007 China International Summer Games | 3,053 | NA | 17.0 | 9.2 | 7.8 |
| 2009 USA International Winter Games | 1,060 | 41.0 | 22.9 | 13.1 | 9.8 |
| 2010 USA National Games | 787 | 36.2 | 32.2 | 18.6 | 13.6 |
| 2011 Greece International Summer Games | 2,660 | 40.9 | 17.1 | 12.1 | 5.0 |
Specific Outcomes for Type and Degree of Hearing Loss
Sensorineural Loss
The data for types of loss (SNHL, C/M) by the degrees of loss (mild, moderate, severe) for 7,560 individuals of the total 9,961 study population provide insights for intervention and treatment needs of adults with ID living in communities at large. As seen in Table 2, the prevalence of bilateral SNHL (10.5% average) is more frequent than unilateral SNHL (2.6% average). Essentially, the degree of bilateral losses is distributed equally between mild/moderate and severe losses, whereas the degree of unilateral losses is mostly mild/moderate in degree. This information certainly has implications for the variety of hearing aids that would be useful for these adults. Most important, though, these results reflect the significant unmet need for amplification of these individuals given that most had no prior experiences with hearing aids.
| Table 2. Type and Degree of Sensorineural Hearing Loss for Specific Special Olympics Events (Herer, 2012) | |||||||
|---|---|---|---|---|---|---|---|
| Events | No. of athletes | Bilateral sensorineural | Unilateral sensorineural | ||||
| Mild (%) | Moderate (%) | Severe (%) | Mild (%) | Moderate (%) | Severe (%) | ||
| 2007 China International Games | 3,053 | 2.0 | 1.6 | 4.2 | 0.7 | 0.1 | 0.6 |
| 2009 USA International Games | 1,060 | 3.5 | 2.5 | 5.0 | 1.2 | 0.3 | 0.6 |
| 2010 USA National Games | 787 | 9.4 | 2.5 | 1.8 | 4.3 | 0.3 | 0.3 |
| 2011 Greece International Games | 2,660 | 2.2 | 2.5 | 4.9 | 0.9 | 0.6 | 0.3 |
Conductive/Mixed Loss
The rates of bilateral and unilateral C/M losses, as seen in Table 3, are quite similar at 4.9% and 4.1%, respectively. The degrees of loss are much the same for the bilateral and unilateral ear conditions as well. They are essentially mild/moderate in degree, typical of hearing loss outcomes from middle ear pathologies. These C/M loss findings for one or both ears of these adults with ID living in the general community emphasize their need for regular ear health examinations and treatment as needed to restore normal hearing.
| Table 3. Type and Degree of Conductive/Mixed Hearing Loss for Specific Special Olympics Event (Herer, 2012) | |||||||
|---|---|---|---|---|---|---|---|
| Events | No. of athletes | Bilateral conductive/mixed | Unilateral conductive/mixed | ||||
| Mild (%) | Moderate (%) | Severe (%) | Mild (%) | Moderate (%) | Severe (%) | ||
| 2007 China International Games | 3,053 | 2.0 | 1.2 | 0.1 | 2.9 | 1.6 | 0.03 |
| 2009 USA International Games | 1,060 | 2.9 | 1.5 | 0.2 | 3.6 | 1.6 | 0 |
| 2010 USA National Games | 787 | 6.9 | 1.4 | 0.4 | 4.4 | 0.5 | 0 |
| 2011 Greece International Games | 2,660 | 1.2 | 1.5 | 0.5 | 0.8 | 0.6 | 0.3 |
Outcomes for Hearing Loss Across Age Decades
Analyses of hearing loss at age decades (10–19, 20–29, 30–39, 40–49, & >50) for 5,713 of the 9,961 individuals with ID of this study show the significant amounts of hearing loss starting in early age decades. The results of Figures 1 and 2 illustrate the trend for increased prevalence of hearing loss with each advancing age decade. Especially notable is the prevalence of hearing loss for the three decades from 20 through 49 years, ranging from 16.8% to 36.1%. A comparison with general non-ID population data for these age decades (Great Britain-1989 and Italy-1996) is presented in Table 4 and reveals remarkable differences. People with ID show losses 3 to 9 times greater depending upon age decade. These data are in concert with the findings of the previously cited study by Meuwese-Jongejeugd et al. (2006) and strongly support the need for annual hearing evaluations for people with ID starting at age 18 and each year thereafter. Such a hearing care protocol is a way to identify the significant numbers of people with ID who experience early onset SNHL or C/M loss and who need immediate follow-up hearing care.
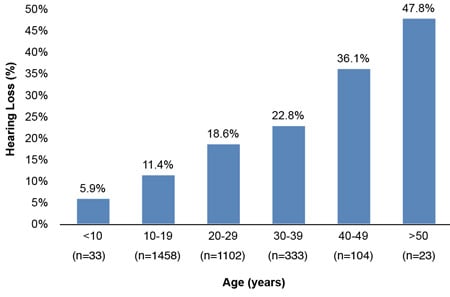
Figure 1. Hearing Loss by Age Decade: 2007 Special Olympics World Summer Games, Shanghai, China (Herer, 2012)
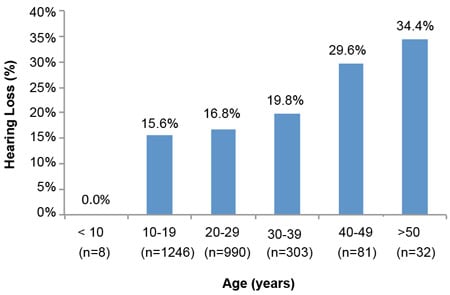
Figure 2. Hearing Loss by Age Decade: 2011 Special Olympics World Summer Games, Athens, Greece (Herer, 2012)
| Table 4. A Comparison of Findings Displayed in Figures 1 & 2 With General Population Data (Herer, 2012) | ||||
|---|---|---|---|---|
| Age Decade | 2007 World Summer Games | 2011 World Summer Games | 1989 Great Britain General Population* | 1996 Italy General Population** |
| 20–29 | 18.6% | 16.8% | 1.8% | 1.9% |
| 30–39 | 22.8% | 19.8% | 2.8% | 3.9% |
| 40–49 | 36.1% | 29.6% | 8.2% | 8.3% |
| 50–59 | 47.8% | 34.4% | 18.9% | 18.7% |
*Davis (1989)
**Quaranta et al. (1996)
Outcomes for Ear Canal Health
Finally, information about ear canal health problems of adults with ID needs to be highlighted in this essay. As shown in Table 1, a high prevalence rate (averaging 41%) of substantial ear wax was identified for the entire 9,961 individuals studied, not just those with hearing loss. This finding underscores the need for improved ear canal hygiene through regular ear examinations for all individuals with ID.
Summary of Findings and Implications for Adults With ID
The results from this 2012 report for the large sample of adults with ID living in the general community highlight the following:
- The prevalence of hearing loss for adults with ID is much higher than for persons in the general population.
- Hearing loss occurs much earlier in age for adults with ID than those in the general population.
- The high prevalence rates of SNHL and C/M loss in persons with ID emphasize their need for hearing aid use and medical care.
- The high prevalence of ear wax in the entire study population with ID emphasizes the need for ear canal examinations on a regular basis for all persons with ID.
- Family members, caregivers, and primary care health care providers must:
- be alerted to the significant hearing care needs of adults with ID;
- be made aware that adults with ID rarely complain about hearing loss and ear canal conditions-that is, adults with ID do not self-identify and therefore existing hearing loss and ear conditions remain undetected;
- serve as advocates for the annual hearing and ear examinations of adults with ID and recognize that these efforts enhance the general physical, social, and cognitive well-being of persons with ID.
Limitations of This Recent Study Leading to Opportunities for Future Research
- The present study could not differentiate results for adults with DS from those with ID not a result of DS. Therefore, the hearing loss rates reported in this study may be influenced by the proportion of adults with DS among the total group tested. Another large sample study of adults with ID living in the general community that does differentiate results for adults with DS from those for adults with ID without DS could provide clarification of any differences. Health care examination and treatment protocols could be influenced by such findings.
- The data reported in Table 1 for international events in years 2005, 2007, 2009, and 2011 were not examined by country of origin for the participants at these events. The health conditions and care in some countries could influence the rate of hearing loss of their adult citizens with ID. Further studies of people with ID living in the general communities of a variety of counties could be helpful in identifying specific care needs in respective countries.
- The degree of ID for the adults in the 2012 report was not determined; although it was hypothesized as being mostly mild, based on observation of their social behavior at the sports events in which they participated. Further study of the hearing of adults with documented degrees of ID and living in their general communities might reveal differences of degree and type of hearing loss by degree of ID and thus influence care planning.
- Data were not available for the present study participants concerning the following characteristics: gender, race, ethnicity, noise exposure, smoking experiences, cardiac status, diabetes, or medication usage. The availability of such information could illuminate similarities or differences concerning rates and types/degrees of hearing loss in people with ID with such characteristics compared with the general population. For example, one study in the literature (Agrawal, Platz, & Niparko, 2008) noted that hearing loss prevalence increased for those in the general population with smoking and noise exposure experiences, as well as cardiovascular risks. Further study of adults with ID with these characteristics could be very revealing and possibly lead to preventive or intervention measures for vulnerable groups within the adult population with ID.
About the Author
Gilbert R. Herer, an audiologist, is director emeritus of Children's Hearing and Speech Center, Children's National Medical Center, and professor emeritus of Pediatrics, George Washington University, Washington, DC. He is currently adjunct professor of audiology, Chapman University, Orange, CA. Dr. Herer is former ASHA president (1989) and was awarded ASHA Honors in 2008. He is also founder/emeritus senior global advisor of healthy hearing for Special Olympics International. Dr. Herer received his bachelor and master of science degrees in communication sciences and disorders from Syracuse University under the mentorship of Dr. Louis M. DiCarlo and received the University's 1998 Outstanding Alumni Award. He completed his PhD degree in audiology at Northwestern University under the mentorship of Dr. Raymond T. Carhart, who is recognized as the Father of Audiology. Dr. Herer's current interests include the delivery of audiology services in a public health model and audiology graduate education of speech-language pathology students. Contact him at gilbert.herer@verizon.net.
References
Agrawal, Y., Platz, E. A., & Niparko, J. K. (2008). Prevalence of hearing loss and differences by demographic characteristics among US adults. Archives of Internal Medicine, 168(14), 1522–1530.
Barr, E., Dungworth, J., Hunter, K., McFarlane, M., & Kubba, H. (2011). The prevalence of ear, nose and throat disorders in preschool children with Down's syndrome in Glasgow. Scottish Medical Journal, 56(2), 98–103.
Davis, A. C. (1989). The prevalence of hearing impairment and reported hearing disability among adults in Great Britain. International Journal of Epidemiology, 18(4), 911–917.
Driscoll, C., Kei, J., Bates, D., & McPherson, B. (2003). Tympanometry and TEOAE testing of children with Down syndrome in special schools. Australian and New Zealand Journal of Audiology, 25(2), 85–93.
Evenhuis, H., van Splunder, J., Vink, M., Weerdenburg, C., van Zanten, B., & Stilma, J. (2004). Obstacles in large-scale epidemiological assessment of sensory impairments in a Dutch population with intellectual disabilities. Journal of Intellectual Disability Research, 48(Pt 8), 708–718.
Evenhuis, H. M. (1995). Medical aspects of ageing in a population with intellectual disability: II. Hearing impairment. Journal of Intellectual Disability Research, 39(Pt 1), 27–33.
Evenhuis, H. M., Theunissen, M., Denkers, I., Verschuure, H., & Kemme, H. (2001). Prevalence of visual and hearing impairment in a Dutch institutionalized population with intellectual disability. Journal of Intellectual Disability Research, 45(Pt 5), 457–464.
Harigai, S. (1994). Longitudinal studies in hearing-impaired children with Down's syndrome. Nihon Jibiinkoka Gakkai Kaiho, 97(12), 2208–2218.
Herer, G. R. (2012). Intellectual disabilities and hearing loss. Communication Disorders Quarterly, 33(4), 252–260. doi: 10.1177/1525740112448214.
Herer, G. R., & Montgomery, J. K. (Eds.). (2006). Special Olympics Healthy Hearing Program: Guidelines for standardized screening procedures, 2nd ed. (pp. 1–90). Washington, DC: Special Olympics.
Hess, C., Rosanowski, F., Eysholdt, U., & Shuster, M. (2006). Hearing impairment in children and adolescents with Down's syndrome. HNO, 54(3), 227–232.
Hildmann, A., Hildmann, H., & Kessler, A. (2002). Hearing disorders in children with Down's syndrome. Laryngorhinootologie, 81(1), 3–7.
Kanamori, G., Witter, M., Brown, J., & Williams-Smith, L. (2000). Otolaryngologic manifestations of Down syndrome. Otolaryngologic Clinics of North America, 33(6), 1285–1292.
Meuwese-Jongejeugd, A., Vink, M., van Zanten, B., Verschuure, H., Eichhorn, E., Koopman, D., … & Evenhuis, H. (2006). Prevalence of hearing loss in 1598 adults with an intellectual disability: Cross-sectional population based study. International Journal ofAudiology, 45, 660–669.
Park, A. H., Wilson, M. A., Stevens, P. T., Harward, R., & Hohler, N. (2012).Identification of hearing loss in pediatric patients with Down syndrome. Otolaryngology-Head & Neck Surgery, 146(1), 135–140.
Quaranta, A., Assennato, G., & Sallustio, V. (1996). Epidemiology of hearing problems among adults in Italy. Scandanavian Audiology Supplement, 42, 9–13.
Shott, S. R., Joseph, A., & Heithaus, D. (2001). Hearing loss in children with Down syndrome. International Journal of Pediatric Otorhinolaryngology, 61(3), 199–205.
Szyfter, W., & Laczkowska-Przybylska, J. (1995). Hearing impairment in children with Down's syndrome. Otolaryngologia polska, 49(5), 436–444.
van Allen, M. I., Fung, J., & Jurenka, S. B. (1999). Health care concerns and guidelines for adults with Down syndrome. American Journal of Medical Genetics, 89(2), 100–110.
Van Buggenhout, G. J., Trommelen, J. C., Schoenmaker, A., De Bal, C., Verbeek, J. J., Smeets, D. F., … & Fryns, J. P. (1999). Down syndrome in a population of elderly mentally retarded patients: genetic-diagnostic survey and implications for medical care. American Journal of Medical Genetics, 85(4), 376–384.
Wilson, D. H., Walsh, P. G., Sanchez, L., Davis, A. C., Taylor, A. W., Tucker, G., & Meagher, I. (1999). The epidemiology of hearing impairment in an Australian adult population. International Journal of Epidemiology, 28(2), 247–252.










