Genetics of Auditory Disorders
October 2013
Danielle Mercer, MS, and Fern Tsien, PhD
Of every 1,000 babies born in the United States, an estimated 3 to 4 will have permanent congenital hearing loss (National Center for Hearing Assessment and Management, 2010). Genetic causes are implicated in roughly half of these cases, with the other half being attributable to environmental causes (Rehm, 2005; Smith, Bale, & White, 2005). Genetic forms of hearing loss can be syndromic, indicating other physical features are seen alongside the hearing loss, or nonsyndromic, meaning hearing loss is the only finding. Among genetic forms of hearing loss, approximately 30% of cases are syndromic and 70% are nonsyndromic (Keats, 2002; Smith et al., 2005). More than 400 genes responsible for hearing loss have been identified (Toriello, Reardon, & Gorlin, 2004); most are part of a syndrome. Identifying the cause of the hearing loss can benefit patients and their families by uncovering other health risks, estimating recurrence risks for future offspring, and indicating other family members who may be at risk. Genes are located on the DNA of individuals. Genes are carried on chromosomes, which are passed down from parent to child. With few exceptions, each cell in the human body contains 46 chromosomes. Egg and sperm cells contain half, or 23 chromosomes, restoring the chromosomal number to 46 in the newly formed zygote. This arrangement specifies an individual will have two copies of every gene: one from each parent. One pair of chromosomes—designated sex chromosomes—confers gender. The remaining 22 pairs, referred to as autosomes, are numbered from 1 to 22, in descending order roughly according to size.
Patterns of Inheritance
Genetic disorders can be inherited in different ways: autosomal dominant, autosomal recessive, X-linked, and mitochondrial patterns of inheritance. In an autosomal dominant pattern of inheritance, a child inherits a normal copy of a gene from one parent and an abnormal gene from the other parent. The abnormal gene dominates the normal gene, so one copy of an abnormal gene is enough to cause an autosomal dominant disorder (Figure 1). Waardenburg syndrome is the most common cause of autosomal dominant syndromic hearing loss, affecting 1 in 42,000 people (Read & Newton, 1997). When a parent has Waardenburg syndrome, each child of that parent has a 50% chance of being affected by Waardenburg syndrome, assuming the other parent does not have the syndrome. The other 50% of offspring in this mating will be unaffected. Typical features of Waardenburg syndrome include sensorineural hearing loss, a white forelock, pale blue or differently colored eyes, and widely spaced eyes. A great deal of variable expressivity exists in Waardenburg syndrome, such that patients may have any combination of features, including normal hearing (de Sousa Andrade et al., 2012). Waardenburg syndrome accounts for approximately 2% of cases of profound congenital hearing loss (de Sousa Andrade et al., 2012). It should be noted that, in rare cases, some autosomal dominant disorders occur due to a new mutation in the child and neither parent has the disorder.
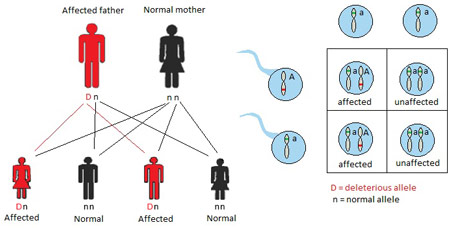
Figure 1. Autosomal dominant inheritance
When a disorder is passed on in an autosomal recessive fashion, two copies of the abnormal gene are required to cause the disorder. Individuals who inherit only one abnormal gene and one normal gene are referred to as carriersand are notaffected by the disorder. Carriers frequently are unaware of their carrier status until they have an affected child. Individuals affected by an autosomal recessive disorder usually are the result of matings between two carriers. Figure 2 demonstrates potential outcomes of such a mating, whereby 25% of offspring are affected by the disorder in question, 50% are unaffected carriers, and 25% are free of the disorder and the abnormal gene. Consanguinity, or the presence of a common ancestor between mates, increases the risk of an autosomal recessive disorder. Usher syndrome demonstrates an autosomal recessive pattern of inheritance. The prevalence of Usher syndrome is 3 to 4 per 100,000 (Ahmed, Riazuddin, Riazuddin, & Wilcox, 2003), with higher prevalence rates in certain populations, including the Acadian population in Louisiana (Ouyang et al., 2003) and the Ashkenazi Jewish population (Guha et al., 2012). The most severe type of Usher syndrome is characterized by congenital profound sensorineural hearing loss and progressive vision loss (Liu et al., 2013). Securing an early diagnosis of Usher syndrome can help patients prepare for their futures. Usher syndrome may account for half of all cases of concurrent deafness/blindness (Yan & Liu, 2010).
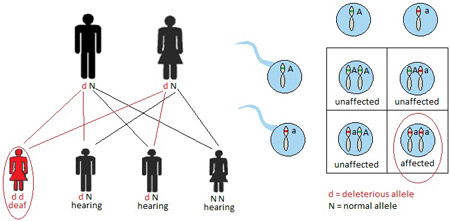
Figure 2. Autosomal recessive inheritance
X-linked, or sex-linked, disorders are inherited through genes on the X chromosome. Males only carry one X chromosome. Therefore, males are usually more susceptible to the clinical symptoms of an X-linked disorder. Although females carry two copies of the X chromosome and single mutation carriers are often unaffected by the disorder, others may show a wide range of clinical symptoms. Figure 3 shows an example in which the mother is an unaffected carrier and the father has a normal copy of the gene. The affected son inherited the abnormal X chromosome from the mother. In X-linked disorders, affected fathers usually pass on the abnormal gene to their daughters, but not to their sons. More than 80% of Alport syndrome patients have an X-linked disorder associated with sensorineural hearing loss, kidney disease, and ocular abnormalities (Kruegel, Rubel, & Gross, 2013; Rheault, 2012).
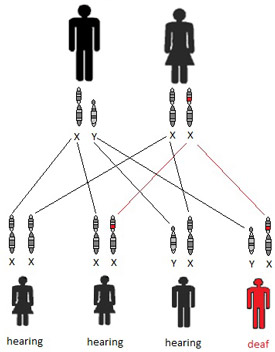
Figure 3. X-linked inheritance
Though mutations in mitochondrial genes account for a small percentage of nonsyndromic hearing loss cases, they are important to note for their mode of inheritance and their association with an unusually high susceptibility to aminoglycoside ototoxicity (Chen, He, Fu, & Dong, 2011). Mitochondria are cell organelles responsible for energy production. They are located outside the nucleus and have their own DNA and genes, separate from the nuclear genes. Mitochondria are found in egg cells, but not in sperm cells, so they are inherited exclusively through the maternal line. A woman with a mitochondrial mutation will pass the mutation to ALL of her children, while a man with a mitochondrial mutation will not pass it on to any of his children. Mitochondrial inheritance is demonstrated in Figure 4. Mutations in the mitochondrial MT-RNR1gene cause an increased susceptibility to aminoglycoside-induced ototoxicity, sometimes causing profound hearing loss after just one dose of aminoglycoside antibiotics (Van Camp & Smith, 2000). Newborn screening for mitochondrial mutations leading to aminoglycoside-induced ototoxicity is not yet available. Genetic testing should be requested, if a maternal inheritance pattern is observed (Figure 4) and the child is affected following administration of aminoglycoside antibiotics. Genetic testing of a mitochondrial mutation can identify other family members or future pregnancies at risk of early-onset substantial hearing loss, thereby allow for preventive measures to be taken. In these cases, parents are advised to avoid the administration of aminoglycoside antibiotics and seek alternative antibiotic treatments.
Figure 4. Mitochondrial inheritance
More than 60 genes implicated in nonsyndromic hearing loss have been identified (Van Camp & Smith, 2013). Approximately 80% of genetic nonsyndromic hearing loss cases are inherited in an autosomal recessive fashion, while nearly 20% demonstrate an autosomal dominant mode of inheritance (Hilgert, Smith, & Van Camp, 2009). X-linked and mitochondrial genes account for a small percentage of cases (Hilgert et al., 2009). The most common gene causing nonsyndromic hearing loss is the GJB2gene, also known as Connexin 26or DFNB1 (Angeli, Lin, & Liu, 2012). The Connexin 26 gene accounts for about half of all autosomal recessive causes of nonsyndromic hearing loss (Kenneson, Van Naarden Braun, & Boyle, 2002). Most of these patients are born to normal-hearing parents who are both carriers of mutations in the same gene (Angeli et al., 2012).
Genetic Testing Techniques
There are many types of genetic tests available that target different portions of the genome. Genetic disorders can be caused by abnormalities in various regions; the method for identifying the genetic defect depends on the type of genetic syndrome or abnormality suspected and, thus, their known genomic defect. From large-scale to small-scale chromosome or DNA changes, genetic disorders may result from a change in the number of chromosomes, deletion or addition of a portion of a chromosomal region, or a mutation of DNA within a single gene.
Cytogenetic (karyotype or chromosome) testing is used to detect chromosomal abnormalities. Changes in the number of chromosomes in each cell are often incompatible with life, because each chromosome contains thousands of genes. Loss or gain of such a large number of genes is very disruptive to development. The most common survivable form of aberrant chromosome number is Down syndrome, which affects 1 in 700 births (Ramia, Musharrafieh, Khaddage, & Sabri, 2013). Down syndrome, also known as Trisomy 21, results from an extra copy of chromosome 21. Individuals with Down syndrome, therefore, have a chromosome count of 47 instead of the usual 46. Down syndrome is a major cause of intellectual disability, and hearing deficits are reported in 34% to 78% of pediatric cases (Raut et al., 2011; Shott, Joseph, & Heithaus, 2001). Hearing loss can be conductive, sensorineural, or mixed (Austeng et al., 2013), and otitis media is very common (Ramia et al., 2013). Most cases of Down syndrome are sporadic, but a small number of cases are inherited (Verma, Lall, & Dua Puri, 2012). Advanced maternal age is a major risk factor (Smith & Visootsak, 2013). Diagnosis is made by chromosome analysis of blood or, prenatally, by chromosome analysis of amniotic fluid or chorionic villus sampling. Samples are examined microscopically to analyze the total number of chromosomes present and to determine which chromosomes are duplicated or missing. Cells are imaged and chromosomes are arranged into a karyotype (see Figure 5). Cytogenetic testing is extremely useful in the diagnosis of trisomies and other chromosomal abnormalities. However, smaller genetic rearrangements may not be visible due to its limited resolution.
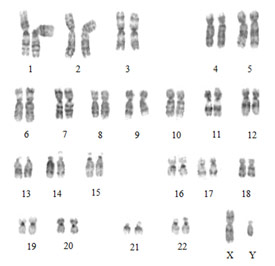
Figure 5. Normal male karyotype
Chromosomes with deletions or duplications too small to be visualized with routine cytogenetic testing can be further analyzed by FISH (fluorescence in situhybridization).FISH testing involves application of a short segment of DNA (probe) coding for the suspected disorder labeled with a fluorescent tag. The fluorescent probe will attach to its complementary sequence on the patient sample. Cells are then analyzed for the presence or absence of the fluorescent probe signal under the microscope. The advantage of FISH is that it is very specific for the diagnosis of microdeletions and microduplications. However, a particular disorder must be suspected so that the correct FISH probe will be used. A list of microdeletion syndromes can be found on the Springer Protocols website (Schwartz & Graf, 2002). An example of a FISH test is shown in Figure 6.
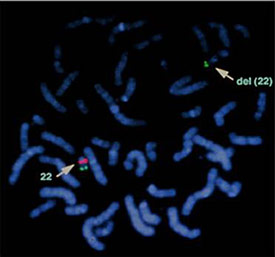
Figure 6. Diagnosis of DiGeorge syndrome using fluorescence in situhybridization (FISH). Green signal: normal control. Red signal: DiGeorge syndrome gene region. Del (22): positive deletion of the DiGeorge gene on chromosome 22.
In recent years, microarray technology has been developed for clinical genetic testing. Microarray comparative genomic hybridization (CGH) compares the DNA from the patient with DNA from a normal control. The DNA samples of the patient and control are labeled with differently colored fluorescent probes and allowed to compete for the binding to the thousands of DNA segments on a glass “chip.” This is then scanned by a laser and the differences between the patient DNA and normal control DNA are analyzed by specialized computer software. Gains and losses of DNA can be visualized by this technology, which gives a broad view of the entire genome; it is, therefore, not necessary to know the exact genetic anomaly when requesting this test. Microarrays can be customized to search for a particular group of disorders. For example, a chip with segments of many different genes associated with hearing loss could be created to test for multiple deafness genes at one time. A limitation of microarrays is that unusual findings may be difficult to interpret as well as differences between a normal genetic variant and a true genetic pathology (Evangelidou et al., 2013).
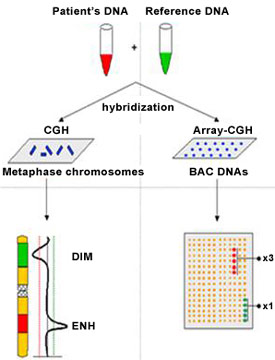
Figure 7. Microarray Comparative Genomic Hybridization (CGH)
Some types of hearing loss are caused by mutations or changes in the DNA that are too small to be detected by the previously mentioned methodologies. Mutation analysis may be done by PCR (polymerase chain reaction). PCR is a technique of amplifying a small segment of DNA to produce millions of copies. This technique generates a large sample of DNA that can be analyzed by visualizing the sample through gel electrophoresis. The smallest genetic change involves replacement of one base pair of DNA with another, termed a point mutation. Finding a point mutation may also require DNA sequencing of the gene in question following PCR. The DNA sequencing technique involves the analysis of each DNA base of the gene region, enabling the detection of substitutions, deletions, or insertions involving a single base. Gene sequencing is the preferred method of testing for Connexin 26 mutations (Figure 8; Angeli et al., 2012).
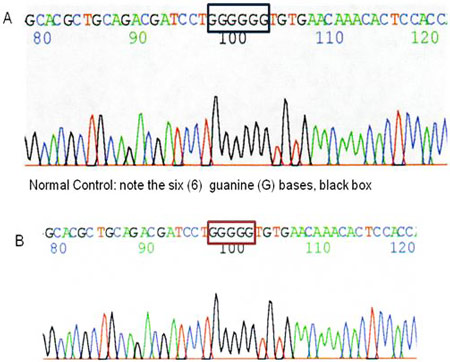
Figure 8.Diagnosis of Connexin 26 mutation by DNA sequencing. Positive results for deafness caused by Connexin 26. Because this type of deafness follows an autosomal recessive inheritance pattern, an affected patient demonstrates a deletion of one guanine (G) base in both Connexin 26 (GJB2) genes. Note the five (5) G bases instead of the normal six (6), red box.
In addition to patient diagnosis, genetic techniques are crucial for hearing loss research. Human genes can be inserted into other organisms to study how they function. Mice are the organisms of choice in the study of deafness genes for the following reasons: (1) the genome and inner ear of the mouse are very similar to that of humans, (2) mice have a short gestation time, and (3) they are relatively easy to selectively mate. Numerous mouse models representing human hearing loss—including models for Usher syndrome and Waardenburg syndrome—(Angeli et al., 2012; Ni et al., 2013) have been developed.
Summary
Many different types of genetic changes can be associated with hearing loss. The type of diagnostic test selected will depend on the type of genetic changes suspected. Clues can be gleaned from the family history and inheritance pattern, but clinicians should remember that a negative family history does not imply that the etiology is not genetic. Genetic testing can allow for early detection of other potential health issues, estimate recurrence risks, and alert other family members who may be affected. Genetic testing should not be undertaken until environmental causes, such as infections and trauma, are ruled out. Audiologists should locate the geneticists in their area and maintain a close relationship with them for optimal care of patients with genetic forms of hearing loss. Information about genetic syndromes, testing, and geneticists in your area can be found at the National Institutes of Health Genetic Testing Registry and Genetests websites.
About the Authors
Danielle Mercer is a third-year doctoral student in the audiology program at the Department of Communication Disorders at LSUHSC. She holds a bachelor’s degree in biology from William Jewell College in Liberty, Missouri, and a master’s degree in dietetics and nutrition from the University of Kansas. Ms. Mercer has over 10 years of laboratory experience in clinical diagnostics and scientific research, including cytogenetics, molecular genetics, leukemia research, and clinical research. She is board certified in cytogenetics by the American Society for Clinical Pathology Board of Registry. She has a strong interest in the genetics of hearing loss and hopes to pursue this facet of audiology. Contact her at dmerc2@lsuhsc.edu.
Fern Tsien is currently a faculty member in the Department of Genetics (School of Medicine) and Department of Communication Disorders (School of Allied Health Professions) at LSUHSC. She teaches medical students, graduate students, clinical laboratory technicians, audiology pre-doctoral students, speech-language pathology students, and audiologists. Dr. Tsien received her PhD degree from the Hayward Genetics Center, Human Genetics Program at the Tulane University School of Medicine. She is currently evaluating the Down syndrome critical region and the effect of speech language therapy on patients with Down syndrome. She is also studying chromosome and epigenetic instability in the DNA methyltransferase 3B-deficient immunodeficiency, centromeric region instability, and facial anomalies (ICF) syndrome and its relationship to cancer-related chromatin instability. Contact her at fmille@lsuhsc.edu.
References
Ahmed, Z. M., Riazuddin, S., Riazuddin, S., & Wilcox, E. R. (2003). The molecular genetics of Usher syndrome. Clinical Genetics, 63, 431–444.
Angeli, S., Lin, X., & Liu, X. Z. (2012). Genetics of hearing and deafness. Anatomical Record, 295, 1812–1829.
Austeng, M. E., Akre, H., Overland, B., Abdelnoor, M., Falkenberg, E. S., & Kvaerner, K. J. (2013). Hearing level in children with Down syndrome at the age of eight. Research in Developmental Disabilities, 34, 2251–2256.
Chen, G., He, F., Fu, S., & Dong, J. (2011). GJB2 and mitochondrial DNA 1555A>G mutations in students with hearing loss in the Hubei province of China. International Journal of Pediatric Otorhinolaryngology, 75, 1156–1159.
de Sousa Andrade, S. M., Monteiro, A. R., Martins, J. H., Alves, M. C., Santos Silva, L. F., Evangelidou P., Alexandrou, A., Moutafi, M., Ioannides, M., Antoniou, P., Koumbaris, ... Patsalis, P. C. (2013). Implementation of high resolution whole genome array CGH in the prenatal clinical setting: Advantages, challenges, and review of the literature. BioMed Research International, 2013, 346762.
Guha S., Rosenfeld, J. A., Malhotra, A. K., Lee, A. T., Gregersen, P. K., Kane, J. M., ... Lencz, T. (2012). Implications for health and disease in the genetic signature of the Ashkenazi Jewish population. Genome Biology, 13, R2.
Hilgert, N., Smith, R. J. H., & Van Camp, G. (2009). Forty-six genes causing nonsyndromic hearing impairment: Which ones should be analyzed in DNA diagnostics? Mutation Research, 681, 189–196.
Keats, B. J. (2002). Genes and syndromic hearing loss. Journal of Communication Disorders, 35, 355–366.
Kenneson, A., Van Naarden Braun, K., & Boyle, C. (2002). GJB2 (Connexin 26) variants and nonsyndromic sensorineural hearing loss: A HuGE review. Genetics in Medicine, 4, 258–274.
Kruegel, J., Rubel, D., & Gross, O. (2013). Alport syndrome- insights from basic and clinical research. Nature Reviews Nephrology, 9, 170–178.
Liu, F., Li, P., Liu, Y., Li, W., Wong, F., Du, R., & ... Liu, M. (2013). Novel compound heterozygous mutations in MYO7A in a Chinese family with Usher syndrome type 1. Molecular Vision, 19, 695–701.
National Center for Hearing Assessment and Management. (2010). Universal newborn hearing screening: Issues and evidence. Retrieved April 28, 2013.
Ni, C., Zhang, D., Beyer, L. A., Halsey, K. E., Fukui, H., Raphael, Y., & ... Hornyak, T. J. (2013). Hearing dysfunction in herterozygous Mitf(Mi-wh)/+ mice, a model for Waardenburg syndrome type 2 and Tietz syndrome. Pigment Cell Melanoma Research, 26, 78–87.
Ouyang, X. M., Hejtmancik, J. F., Jacobson, S. G., Xia, X. J., Li, A., Du, L. L., & ... Liu, X. Z. (2003). USH1C: a rare cause of USH1 in a non-Acadian population and a founder effect of the Acadian allele. Clinical Genetics, 63, 150–153.
Quadros, J. M., & Ribeiro, C. A. (2012). Cochlear implant rehabilitation outcomes in Waardenburg syndrome children. International Journal of Pediatric Otorhinolaryngology, 76, 1375–1378.
Ramia, M., Musharrafieh, U., Khaddage, W., & Sabri, A. (2013). Revisiting Down syndrome from the ENT perspective: Review of literature and recommendations [published ahead of print, May 21]. European Archives of Oto-Rhino-Laryngology.
Raut, P., Sriram, B., Yeoh, A., Hee, K. Y., Lim, S. B., & Daniel, M. L. (2011). High prevalence of hearing loss in Down syndrome at first year of life. Annals of the Academy of Medicine, Singapore, 40, 493–498.
Read, A. P., & Newton, V. E. (1997). Waardenburg syndrome. Journal of Medical Genetics, 34, 656–665.
Rehm, H. L. (2005). A genetic approach to the child with sensorineural hearing loss. Seminars in Perinatology, 29,173–181.
Rheault, M. N. (2012). Women and Alport syndrome. Pediatric Nephrology, 27, 41–46.
Schwartz, S., & Graf, M. D. (2002). Microdeletion syndromes: Characteristics and diagnosis . In Y. S. Fan (Ed.), Molecular cytogenetics: Protocols and applications (pp. 275–290). Retrieved October 3, 2013.
Shott, S. R., Joseph, A., & Heithaus, D. (2001). Hearing loss in children with Down syndrome. International Journal of Pediatric Otorhinolaryngology, 61, 199–205.
Smith, M., & Visootsak, J. (2013). Noninvasive screening tools for Down syndrome: a review. International Journal of Women’s Health, 5, 125–131.
Smith, R. J., Bale, Jr., J. F., & White, K. R. (2005). Sensorineural hearing loss in children. TheLancet, 365, 879–890.
Toriello, H. V., Reardon, W., & Gorlin, R. J. (2004). Hereditary hearing loss and its syndromes.New York, NY: Oxford University Press.
Van Camp, G., & Smith, R. J. H. (2000). Maternally inherited hearing impairment. Clinical Genetics, 57, 409–414.
Van Camp, G., & Smith, R. (2013). Hereditary hearing loss homepage.Retrieved July 31, 2013.
Verma, I. C., Lall, M., & Dua Puri, R. (2012). Down syndrome in India—diagnosis, screening, and prenatal diagnosis. Clinics in Laboratory Medicine, 32, 231–248.
Yan, D., & Liu, X. Z. (2010). Genetics and pathological mechanisms of Usher syndrome. Journal of Human Genetics, 55, 327–335.










